Abstract
Purpose
There is increasing evidence that disease and therapy-related symptoms frequently co-occur in so-called symptom clusters (SCs), which may significantly impair quality of life in patients with cancer. Although psychosocial resources may play pivotal roles in maintaining or improving quality of life, they have been neglected in SC research. Therefore, we aim to identify SCs and their relative impact on quality of life when psychosocial resources are accounted for.
Methods
Patients with cancer (n = 304) undergoing chemotherapy or chemo-radiation therapy participated in a cross-sectional survey consisting of measures assessing symptoms, quality of life, resilience, treatment-specific optimism (TSO), and social support. Exploratory factor analyses and multiple regression analyses were used to identify SCs and significant explanatory variables of overall quality of life.
Results
Fatigue-pain, anxiety-depression, cancer therapy-related toxicity, and nausea-vomiting clusters were identified. In our final model, the fatigue-pain cluster (β = − 0.41, p < 0.001), nausea-vomiting cluster (β = − 0.28, p < 0.001), TSO (β = 0.21, p < 0.001), and receiving chemo-radiation treatment (β = − 0.11, p = 0.03) accounted for 44% of variance in overall quality of life. However, the identified SCs explained quality of life in patients with varying levels of TSO to a different extent.
Conclusions
Our findings indicate that the TSO of patients may be a major factor to consider in managing SCs, because—depending on its level—different SCs and even clusters encompassing comparatively less distressing symptoms (i.e., cancer therapy-related toxicities) may strongly affect quality of life.
Similar content being viewed by others
Introduction
Despite steady advances in cancer research and increasingly effective treatment options available to patients, cancer treatment is still associated with various undesirable side effects and symptoms [1, 2], which may result in significant distress, functional impairment, and reduced quality of life [3]. Although most clinical studies in symptom research focus primarily on individual symptoms, the isolated occurrence of symptoms in patients with advanced cancer is rather seldom observed in practice [1]. Indeed, there is increasing evidence that symptoms in patients with cancer occur frequently, in a non-random distribution, in groups or so-called symptom clusters [4,5,6]. A symptom cluster (SC) is defined as two or more interrelated symptoms that occur concurrently and may or may not share the same etiology [7]. For example, nausea-vomiting and anxiety-depression SCs are commonly identified in cancer research [5], calling for thorough assessment of co-occurring symptoms and coordinated symptom management strategies to prevent negative outcomes such as poor quality of life [8].
However, critical patient-reported outcomes such as quality of life by far depend not only on symptoms but also on several psychosocial resources that have been consistently linked with quality of life. In cancer patients, the capacity for resilience—defined as a dynamic process of adaptation to adversity associated with the diagnosis and disease as well as treatment-related physical and psychosocial symptoms [9]—was reported to be a factor associated with less anxiety [10, 11] and depression [10,11,12,13] as well as a higher physical activity level [14] and, ultimately, a better quality of life [11, 15]. Social support (emotional, informational, and instrumental assistance by others) is another critical concept to be considered, both contributing to resilience and helping patients maintain an acceptable level of quality of life by itself [16]. Social support has been extensively investigated for its association with positive health outcomes in general [17] and in patients with cancer [18], when it predicted symptoms such as pain and depression [19] and was associated with better health-related quality of life [20]. Similarly, optimism contributes to resilience [9] and links among an optimistic disposition, well-being, and health behavior are well documented in the literature [21]. Treatment-specific optimism (i.e., optimistic expectations concerning the current treatment) may enable patients with cancer to cope more effectively with their experiences and has been shown to be associated with better mental health outcomes [22, 23] and better quality of life [22, 24]. Correspondingly, accounting for psychosocial resources in SC research might offer new insights and potential pathways for symptom management interventions aimed at maintaining or improving quality of life in cancer patients.
However, to our knowledge, the relative impact of SCs and psychosocial resources, particularly resilience and treatment-specific optimism (TSO), on quality of life in cancer patients undergoing treatment has not been studied yet. Therefore, we identified SCs and investigated which of these clusters explain most of the variation in quality of life in patients with cancer undergoing treatment, while accounting for the patients’ psychosocial resources.
Methods
Study design, sample, and setting
This study is based on data from a cross-sectional quantitative survey in several day care units and inpatient departments treating patients with cancer at a large university medical center in Vienna (Austria). Over a 2-week period in May 2014, study participation was offered to all adult (≥ 18 years) inpatients and day care unit patients diagnosed with cancer undergoing chemotherapy, radiotherapy, or chemo-radiation therapy who were judged to be mentally and physically able to participate in the study by their medical or nursing staff (convenience sample). The ethics commission of the Medical University of Vienna reviewed and approved the study protocol (no. 1223/2014). Participation was strictly voluntary. Each patient was informed about the study aims and procedures orally and in writing before informed consent was obtained.
Instruments
Quality of life was assessed using the author-approved German translation of the Rotterdam Symptom Checklist (RSCL). The 39-item questionnaire measures four domains: physical symptom distress, psychological distress, activity level, and overall quality of life. Symptom items are rated on a 4-point scale, but overall quality of life is rated on a 7-point scale. Higher values indicate greater distress and better quality of life, respectively. Psychometric properties of the German translation of the RSCL are adequate, and internal consistency of the physical and psychological distress scales (Cronbach’s α = 0.85) is high [25].
Resilience was measured using the author-approved German translation of the 10-item Connor-Davidson Resilience Scale (CD-RISC 10). It is a generic, unidimensional scale reflecting the ability to bounce back from a variety of challenges arising in life (i.e., stress coping ability), such as illness, emotional pressure, and painful feelings. Items are rated on a 5-point scale and are summated to obtain a score ranging from 0 to 40, with higher scores reflecting greater resilience [26, 27]. Psychometric properties of the German translation of the scale are acceptable, and internal consistency (Cronbach’s α = 0.84) is high [28].
Perceived adequacy of social support from family, friends, and significant others was assessed using the 12-item Multidimensional Scale of Perceived Social Support (MSPSS) [29]. We transformed the original 7-point scale to a 5-point rating scale to provide a consistent response format throughout the questionnaire. Correspondingly, cumulative scores ranged from 12 to 60, with higher scores indicating higher perceived adequacy of social support. Construct validity and internal consistency (Cronbach’s α = 0.88) of the original scale are adequate [29], and internal consistency of our adapted German translation was slightly higher (Cronbach’s α = 0.91) in this study.
Treatment-specific optimism (TSO) was assessed using a 10-item scale originally adapted by Cohen and colleagues for use in clinical cancer trials. Items relate to positive outcome expectancies (e.g., “the treatment will cure me”), optimistic bias (e.g., “having better success on treatment than other patients”), and confident emotions (e.g., “feeling confident about the treatment”) and are rated on a 5-point scale. Item scores are summated to obtain a total score ranging from 10 to 50, with higher scores indicating greater optimism regarding the treatment received. Internal consistency of the original scale (Cronbach’s α = 0.78) [23] and our German translation (Cronbach’s α = 0.79) is similarly adequate.
Because no translations were available, the MSPSS and TSO were translated into German (forward and backward) and culturally adapted, following the guidelines of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for the present study [30].
Statistical analysis
All survey data were managed and analyzed with SPSS version 22.0 (IBM Corp., Armonk, NY) using descriptive statistics. Differences in group means were examined using one-way ANOVAs with subsequent Games-Howell post hoc tests. Associations between psychosocial variables and overall quality of life were examined using Spearman’s rank correlation. Missing values were tested with Little’s missing completely at random test. Data missing at random was not imputed (available case analysis), considering the relatively large sample size. P values < 0.05 were considered significant and p values < 0.001 as highly significant. Data were checked for normality by using the Kolmogorov-Smirnov test, and subsequent analysis was conducted accordingly.
Identification of symptom clusters
We conducted exploratory factor analyses (EFA) of the 30 symptom-specific variables in the RSCL. To identify SCs potentially sharing underlying biopsychosocial mechanisms, we used principal axis factoring. Because clusters were expected to have non-zero correlations with one another, the oblique rotation method oblimin was employed in these analyses [31]. The number of extracted factors was estimated based on the scree plot and parallel analysis of eigenvalues. Cross-loadings of symptoms on factors (> 0.32) were expected [32] and permitted. The best factor model was determined by the following criteria: (1) at least three variables loading (≥ ± 0.40) on each factor, (2) acceptable internal consistency (Cronbach’s α ≥ 0.7) of factors, and (3) clinical and theoretical plausibility of factors (symptoms likely to co-occur and to represent distinct symptom clusters). Factorability, i.e., anti-image correlations, the Kaiser-Meyer-Olkin (KMO) criterion (> 0.5), and Bartlett’s test of sphericity, was also considered. Analysis for each factor model was conducted in a stepwise fashion, in which variables not loading (pattern coefficient < 0.32) on any factors and variables not sufficiently correlated (structure coefficient < 0.4, i.e., at least 16% of symptom variance explained) with their respective factor(s) [33] were gradually removed in each model.
Symptom clusters and psychosocial resources as explanatory variables of overall quality of life
For further analysis, we operationalized SCs as the mean of the summated symptom scores for each identified SC. Explanatory variables of overall quality of life (RSCL, dependent variable) were identified with stepwise backward multiple regression analyses, using the SCs and psychosocial resources as well as age, (dummy coded) variables for gender, education, household income, disease status, and treatment modality as independent variables. For all regression analyses, variables remaining in the final models were considered significant explanatory variables of overall quality of life.
Results
Three hundred four patients provided data for this analysis. Clinical and sociodemographic variables of the sample are summarized in Table 1. The patients ranged in age from 18 to 88 years (M = 57.4). The majority of study participants were female (59%), either married or living with a partner (67%), educated beyond compulsory education (90%), and undergoing chemotherapy without concurrent radiation (75%) for an advanced (invasive, recurrent, or metastatic tumor) cancer (71.2%).
Identification of symptom clusters
We ultimately extracted four factors from our data according to the criteria previously mentioned. Individual scores (mean, median, standard deviation, and response categories) for the symptoms constituting the extracted SCs are given in Table 2, with tiredness being rated as the most distressing symptom. One variable (depressed mood) loaded almost equally (with reversed signs) on two factors, but was retained for conceptual reasons and low resulting collinearity. All factor loadings are shown in Table 3.
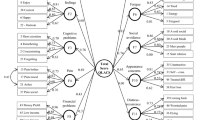
Factor 1 is interpreted as the fatigue-pain cluster. The variables “tiredness” (0.63) and “lack of energy” (0.63) had the highest loadings on this factor, followed by “low back pain” (0.59) and “sore muscles” (0.51). The variables “shortness of breath” (0.47) and “depressed mood” (0.43) had lower loadings. Factor 2 is interpreted as the anxiety and depression cluster, which corresponds to the psychological distress scale of the RSCL. The variables “despairing about the future” (− 0.79), “anxiety” (− 0.79), and “worrying” (− 0.79) loaded equally high on this factor, followed by “nervousness” (− 0.71) and “tension” (− 0.68). Lower factor loadings were obtained for “depressed mood” (− 0.4) and “irritability” (− 0.37). Of note, variable “depressed mood” was retained in the model despite loading on both factor 1 and factor 2 (see “Discussion”). Factor 3 is interpreted as the nausea and vomiting cluster. The variables “nausea” (− 0.8) and “vomiting” (− 0.71) had the highest loadings on this factor, followed by “lack of appetite” (− 0.58). Factor 4 was interpreted as the cancer therapy-related toxicity cluster. The variables “sore mouth/pain when swallowing” (0.56), “tingling hands or feet” (0.48), “loss of hair” (0.44), and “burning/sore eyes” (0.43) had the highest loadings on this factor, followed by “difficulty concentrating” (0.38) and “dry mouth” (0.37).
Internal consistency of factor 1 (Cronbach’s α = 0.8) and factor 2 (Cronbach’s α = 0.87) was high, whereas it was acceptable for factor 3 (Cronbach’s α = 0.73) and factor 4 (Cronbach’s α = 0.68). Of note, neither removing the cross-loading variable “depressed mood” nor removing any variables from factor 4 (with the lowest Cronbach’s α) increased the internal consistency of the respective factors.
Symptom clusters and psychosocial resources as explanatory variables of overall quality of life
Significant positive correlations between quality of life and TSO (r = 0.33, p < 0.001) as well as resilience (r = 0.27, p < 0.001) were observed, but not between quality of life and social support (M = 54.69, SD = 6.96; p > 0.05), which was therefore removed from our regression models. Explanatory variables of overall quality of life (M = 4.92, SD = 1.38) were identified with multiple regression analysis using the identified SCs, TSO (M = 28.98, SD = 6.24), and resilience (M = 29.45, SD = 7.07) as independent variables while controlling for age, gender, education, household income, disease status, and treatment modality.
In the final model (R2 = 0.44, F (4,220) = 42,86, p < 0.001), the fatigue-pain cluster (β = − 0.41, p < 0.001), nausea-vomiting cluster (β = − 0.28, p < 0.001), TSO (β = 0.21, p < 0.001), and receiving chemo-radiation treatment (β = − 0.11, p = 0.03) were retained as significant explanatory variables of overall quality of life (Fig. 1).
Symptom clusters as explanatory variables of overall quality of life in patients with low/medium/high treatment-specific optimism
Because TSO was the only psychosocial variable to remain significant in our final model, we conducted separate analyses to investigate this relationship further. For this purpose, we divided our sample into three subgroups of patients scoring low (< 26), medium (26–32), and high (≥ 33) on TSO (tertiles). Significant differences in quality of life by TSO were determined by a one-way ANOVA (F(2,291) = 21.17, p < 0.001). A Games-Howell post hoc test showed that patients scoring high on TSO reported significantly higher quality of life (M = 5.56, SD = 1.14) than patients with either low (M = 4.41, SD = 1.38, p < 0.001) or medium TSO (M = 4.7, SD = 1.36, p < 0.001). No significant difference in quality of life (p > 0.05) between the low-TSO and medium-TSO groups was found.
For all three subgroups, explanatory variables of overall quality of life were identified with multiple regression analyses as previously described (Fig. 2). For patients scoring low on TSO, the nausea-vomiting cluster (β = − 0.44, p = 0.000), the anxiety-depression cluster (β = − 0.31, p = 0.007), and receiving chemo-radiation treatment (β = − 0.25, p = 0.03) were retained as significant explanatory variables of overall quality of life in the final model (R2 = 0.31, F(3,57) = 8.34, p < 0.001). For patients with medium TSO (R2 = 0.36, F(2,78) = 21.57, p < 0.001), the anxiety-depression cluster (β = − 0.4, p < 0.001) and nausea-vomiting cluster (β = − 0.32, p < 0.001) were retained as significant explanatory variables of overall quality of life. For patients scoring high on TSO, the fatigue-pain cluster (β = − 0.46, p < 0.001) and cancer therapy-related toxicity cluster (β = − 0.31, p = 0.006) were retained as significant explanatory variables of quality of life (R2 = 0.49, F(2,76) = 37.12, p < 0.001).
Of note, significant differences in intensity of symptom clusters (symptom distress) by TSO were found for the fatigue-pain (F(2,264) = 6.34, p = 0.002), anxiety-depression (F(2,256) = 19.05, p < 0.001), and nausea-vomiting clusters (F(2,281) = 6.56, p = 0.002), but not for the cancer therapy-related toxicity cluster (p > 0.05). A Games-Howell post hoc test showed that patients scoring high on TSO were significantly less distressed by the fatigue-pain cluster (M = 1.89, SD = 0.64) than patients in the medium (M = 2.12, SD = 0.62, p = 0.029) and low (M = 2.21, SD = 0.56, p = 0.002) TSO groups. The same is true when comparing the anxiety-depression cluster in the high-TSO and low-TSO groups (M = 1.65, SD = 0.58 vs. M = 2.22, SD = 0.67, p < 0.001) and when comparing the medium-TSO and low-TSO groups (M = 1.83, SD = 0.54 vs. M = 2.22, SD = 0.67, p < 0.001). Patients with high TSO were also less affected by the nausea-vomiting cluster (M = 1.49, SD = 52) compared to those of both the medium-TSO (M = 1.79, SD = 0.69, p = 0.001) and low-TSO groups (M = 1.73, SD = 0.67, p = 0.023). No further significant group differences (p > 0.05) were observed.
Discussion
The present study identified four distinct symptom clusters in a heterogeneous sample of cancer patients undergoing chemotherapy and/or radiotherapy. These are, in order of descending average symptom distress, the following: fatigue-pain, anxiety-depression, cancer therapy-related toxicity, and nausea-vomiting clusters. These clusters conform to findings of recent international studies, which frequently report SCs of identical or very similar composition [34], except for the cancer therapy-related SC. However, because composition of these clusters may vary by factors such as diagnoses and treatment modalities [8], distinct but varying sets of specific treatment-related SCs are to be expected rather than consistent therapy-related SCs across study populations.
We found the variable “depressed mood” to load almost equally on both the fatigue-pain and anxiety-depression clusters. Cross-loading items are potentially problematic when identifying symptom clusters; per definition, clusters should be rather independent from each other. However, we did not remove the cross-loading variable because the cross-loading can be meaningfully explained. On the one hand, the German translation of “depressed mood” (“Niedergeschlagenheit”) might carry both a physical (as in being the result of feeling exhausted) and psychological connotation (as in feeling depressed) for some patients, although the latter interpretation will be more common. On the other hand, the experience of fatigue is not limited to physical sensations. Rather, affective manifestations of fatigue are also to be expected, including feelings of sadness and anxiety [35], which are consequently also assessed in multidimensional fatigue scales [36, 37]. Apart from overlapping symptom experiences, it is equally plausible that depression and fatigue occur separately and simultaneously, while being associated with each other [38, 39]. In each case, we found it highly warranted to retain the “depressed mood” item in two symptom clusters.
In our total sample, overall quality of life was significantly explained by the fatigue-pain cluster, nausea-vomiting cluster, and TSO, as well as receiving concomitant chemoradiotherapy. Both SCs were previously shown to impair quality of life significantly in larger samples of patients with cancer; however, an emotional SC (including depression) was found to be the strongest predictor of quality of life [40]. This is—to some degree—in contrast with our findings, because we found that by accounting for TSO, the anxiety-depression cluster no longer significantly explained quality of life. Thus, treatment-related expectations may in fact strongly influence quality of life ratings, even slightly more so than the treatment modality received by the patients itself. Yet, being treated with concurrent chemotherapy and radiotherapy remained a significant explanatory variable of quality of life in our total sample. This indicates that additional burden may accompany more intensive treatment modalities [41].
We found higher TSO and resilience were both associated with better overall quality of life, which is in line with previous research on both TSO [22, 24] and resilience [11, 15] in patients with cancer undergoing treatment and cancer survivors. We can report neither a significant association of social support and quality of life nor a quadratic effect (i.e., no additional benefits for the patient past a threshold level of social support). Yet, only TSO remained in our final regression model, indicating that resilience may explain comparatively less variation in quality of life when TSO is accounted for. We mainly attribute this to differences in the operationalization of both concepts. Resilience was assessed as “stress coping ability” [27] without referring to any specific context, whereas items measuring TSO explicitly refer to the patient’s expected treatment experiences. For example, patients may perceive their resilience to be high in general but may not be equally confident to cope well with their current treatment.
In addition, we found that SCs significantly explained quality of life in patients with varying levels of TSO to a different extent. Treatment modality may most strongly affect quality of life ratings in patients with comparatively pessimistic treatment-related expectations while otherwise explaining less variation in quality of life. Similarly, nausea-vomiting and anxiety-depression clusters are comparatively not only rated as more distressing by patients with low to medium levels of TSO, they also explain quality of life ratings in these patient groups better than in patients with highly optimistic expectations toward their treatment. In contrast, fatigue-pain and cancer therapy-related SCs may particularly affect quality of life in patients with highly optimistic expectations toward their treatment. This is observed although these patients are, in comparison with their less optimistic counterparts, equally bothered by cancer therapy-related toxicities and even less bothered by the fatigue-pain cluster.
Limitations
We acknowledge several limitations of this study. Due to the cross-sectional nature of this study, we could not establish causality between the variables we investigated, and although we controlled for disease- and treatment-specific variables in our analyses, specific subgroups of patients may naturally experience different SCs. However, the question remains open of whether SCs should be evaluated in homogeneous or heterogeneous samples [42]. Furthermore, although we used factor analysis and specific criteria delineated in the “Methods” section to identify SCs in our sample, we do acknowledge the value of different conceptual and methodological approaches in SC research [43].
Conclusions
Symptom management aimed at maintaining or improving quality of life in patients with cancer requires comprehensive and carefully coordinated assessment and management strategies, because symptoms frequently co-occur in SCs. Our findings indicate that the TSO of patients may be a major factor to consider in achieving this goal, because—depending on its level—different SCs and even clusters encompassing symptoms that are comparatively rated as least distressing (i.e., cancer therapy-related toxicities) may strongly affect quality of life. Thus, being aware of treatment expectations might facilitate the interpretation of symptom distress assessments and should be accounted for in symptom management and patient counseling alongside other important psychosocial resources such as resilience. Although longitudinal studies are required to establish these findings, they may illustrate an important mechanism affecting quality of life in patients with cancer undergoing treatment that may also be partially (confidence to manage side effects and overly negative expectations) amenable to interventions.
References
Fan G, Filipczak L, Chow E (2007) Symptom clusters in cancer patients: a review of the literature. Curr Oncol 14(5):173–179
Miaskowski C, Dodd M, Lee K (2004) Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr 2004(32):17–21. https://doi.org/10.1093/jncimonographs/lgh023
Kroenke K, Johns SA, Theobald D, Wu J, Tu W (2013) Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Support Care Cancer 21(3):765–773. https://doi.org/10.1007/s00520-012-1578-5
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14(8):831–836. https://doi.org/10.1007/s00520-005-0899-z
Kirkova J, Aktas A, Walsh D, Davis MP (2011) Cancer symptom clusters: clinical and research methodology. J Palliat Med 14(10):1149–1166. https://doi.org/10.1089/jpm.2010.0507
Kirkova J, Walsh D, Aktas A, Davis MP (2010) Cancer symptom clusters: old concept but new data. Am J Hosp Palliat Care 27(4):282–288. https://doi.org/10.1177/1049909110364048
Kim HJ, McGuire DB, Tulman L, Barsevick AM (2005) Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 28(4):270–282 quiz 283-274
Kwekkeboom KL (2016) Cancer symptom cluster management. Semin Oncol Nurs 32(4):373–382. https://doi.org/10.1016/j.soncn.2016.08.004
Eicher M, Matzka M, Dubey C, White K (2015) Resilience in adult cancer care: an integrative literature review. Oncol Nurs Forum 42(1):E3–E16. https://doi.org/10.1188/15.onf.e3-e16
Min JA, Yoon S, Lee CU, Chae JH, Lee C, Song KY, Kim TS (2013) Psychological resilience contributes to low emotional distress in cancer patients. Support Care Cancer 21(9):2469–2476. https://doi.org/10.1007/s00520-013-1807-6
Schumacher A, Sauerland C, Silling G, Berdel WE, Stelljes M (2013) Resilience in patients after allogeneic stem cell transplantation. Support Care Cancer 22:487–493. https://doi.org/10.1007/s00520-013-2001-6
Gotay CC, Ransom S, Pagano IS (2007) Quality of life in survivors of multiple primary cancers compared with cancer survivor controls. Cancer 110(9):2101–2109. https://doi.org/10.1002/cncr.23005
Sharpley CF, Wootten AC, Bitsika V, Christie DR (2013) Variability over time-since-diagnosis in the protective effect of psychological resilience against depression in Australian prostate cancer patients: implications for patient treatment models. Am J Mens Health 7(5):414–422. https://doi.org/10.1177/1557988313477126
Matzka M, Mayer H, Köck-Hódi S, Moses-Passini C, Dubey C, Jahn P, Schneeweiss S, Eicher M (2016) Relationship between resilience, psychological distress and physical activity in cancer patients: a cross-sectional observation study. PLoS One 11(4):e0154496. https://doi.org/10.1371/journal.pone.0154496
Gotay CC, Isaacs P, Pagano I (2004) Quality of life in patients who survive a dire prognosis compared to control cancer survivors. Psycho-Oncology 13(12):882–892. https://doi.org/10.1002/pon.808
Arora NK, Finney Rutten LJ, Gustafson DH, Moser R, Hawkins RP (2007) Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psycho-Oncology 16(5):474–486. https://doi.org/10.1002/pon.1084
Cohen S, Gottlieb B, Underwood L (2000) Social relationships and health. In: Cohen S, Underwood L, Gottlieb B (eds) Social support measurement and intervention: a guide for health and social scientists. Oxford University Press, New York, p 368
Tamagawa R, Garland S, Vaska M, Carlson LE (2012) Who benefits from psychosocial interventions in oncology? A systematic review of psychological moderators of treatment outcome. J Behav Med 22:22
Hughes S, Jaremka LM, Alfano CM, Glaser R, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE 3rd, Malarkey WB, Kiecolt-Glaser JK (2014) Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology 42:38–44. https://doi.org/10.1016/j.psyneuen.2013.12.016
Leung J, Pachana NA, McLaughlin D (2014) Social support and health-related quality of life in women with breast cancer: a longitudinal study. Psycho-Oncology 23(9):1014–1020. https://doi.org/10.1002/pon.3523
Glaesmer H, Hoyer J, Klotsche J, Herzberg PY (2008) Die deutsche Version des Life-Orientation-Tests (LOT-R) zum dispositionellen Optimismus und Pessimismus. Z Gesundheitspsych 16(1):26–31. https://doi.org/10.1026/0943-8149.16.1.26
de Moor JS, de Moor CA, Basen-Engquist K, Kudelka A, Bevers MW, Cohen L (2006) Optimism, distress, health-related quality of life, and change in cancer antigen 125 among patients with ovarian cancer undergoing chemotherapy. Psychosom Med 68(4):555–562. https://doi.org/10.1097/01.psy.0000222379.71389.91
Cohen L, de Moor C, Amato RJ (2001) The association between treatment-specific optimism and depressive symptomatology in patients enrolled in a phase I cancer clinical trial. Cancer 91(10):1949–1955
Milbury K, Tannir NM, Cohen L (2011) Treatment-related optimism protects quality of life in a phase II clinical trial for metastatic renal cell carcinoma. Ann Behav Med 42(3):313–320. https://doi.org/10.1007/s12160-12011-19301-12161
de Haes JCJM, Olschewski M (1998) Quality of life assessment in a cross-cultural context: use of the Rotterdam Symptom Checklist in a multinational randomised trial comparing CMF and Zoladex (Goserlin) treatment in early breast cancer. Ann Oncol 9(7):745–750
Campbell-Sills L, Stein MB (2007) Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): validation of a 10-item measure of resilience. J Trauma Stress 20(6):1019–1028
Connor KM, Davidson JR (2003) Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18(2):76–82. https://doi.org/10.1002/da.10113
Sarubin N, Gutt D, Giegling I, Bühner M, Hilbert S, Krähenmann O, Wolf M, Jobst A, Sabaß L, Rujescu D, Falkai P, Padberg F (2015) Erste Analyse der psychometrischen Eigenschaften und Struktur der deutschsprachigen 10- und 25-Item Version der Connor-Davidson Resilience Scale (CD-RISC). Z Gesundheitspsychol 23(3):112–122. https://doi.org/10.1026/0943-8149/a000142
Zimet GD, Dahlem NW, Zimet SG, Farley GK (1988) The multidimensional scale of perceived social support. J Pers Assess 52(1):30–41. https://doi.org/10.1207/s15327752jpa5201_2
Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, Erikson P (2005) Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 8(2):94–104. https://doi.org/10.1111/j.1524-4733.2005.04054.x
Skerman HM, Yates PM, Battistutta D (2012) Identification of cancer-related symptom clusters: an empirical comparison of exploratory factor analysis methods. J Pain Symptom Manag 44(1):10–22. https://doi.org/10.1016/j.jpainsymman.2011.07.009
de Haes JCJM, Olschewski M, Fayers P, Visser MRM, Cull A, Hopwood P, Sanderman R (1996) Measuring the quality of life of cancer patients with the Rotterdam Symptom Checklist (RSCL): a manual. Northern Centre for Healthcare Research (NCH), University of Groningen, Groningen
Yates P, Miaskowski C, Cataldo JK, Paul SM, Cooper BA, Alexander K, Aouizerat B, Dunn L, Ritchie C, McCarthy A, Skerman H (2015) Differences in composition of symptom clusters between older and younger oncology patients. J Pain Symptom Manag 49(6):1025–1034. https://doi.org/10.1016/j.jpainsymman.2014.11.296
Dong ST, Butow PN, Costa DS, Lovell MR, Agar M (2014) Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manag 48(3):411–450. https://doi.org/10.1016/j.jpainsymman.2013.10.027
Glaus A (1998) Fatigue in patients with cancer. Analysis and assessment. Recent Results Cancer Res 145:I-XI, 1-172
Stein KD, Jacobsen PB, Blanchard CM, Thors C (2004) Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manag 27(1):14–23
Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, Hosaka T, Uchitomi Y (2000) Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manag 19(1):5–14
Donovan KA, Jacobsen PB (2010) The fatigue symptom inventory: a systematic review of its psychometric properties. Support Care Cancer 19(2):169–185. https://doi.org/10.1007/s00520-010-0989-4
Brown LF, Kroenke K (2009) Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics 50(5):440–447. https://doi.org/10.1176/appi.psy.50.5.440
Dong ST, Costa DS, Butow PN, Lovell MR, Agar M, Velikova G, Teckle P, Tong A, Tebbutt NC, Clarke SJ, van der Hoek K, King MT, Fayers PM (2016) Symptom clusters in advanced cancer patients: an empirical comparison of statistical methods and the impact on quality of life. J Pain Symptom Manag 51(1):88–98. https://doi.org/10.1016/j.jpainsymman.2015.07.013
Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, Lu C, Garden AS, Morrison WH, Cleeland CS, Gunn GB (2014) Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 120(13):1975–1984. https://doi.org/10.1002/cncr.28672
Molassiotis A, Wengstrom Y, Kearney N (2010) Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manag 39(5):847–858. https://doi.org/10.1016/j.jpainsymman.2009.09.012
Xiao C (2010) The state of science in the study of cancer symptom clusters. Eur J Oncol Nurs 14(5):417–434. https://doi.org/10.1016/j.ejon.2010.05.011
Acknowledgements
Open access funding provided by University of Vienna. We thank the patients for sharing their valuable time, effort, and insights during a difficult time in their lives. We are grateful to the staff of the Vienna General Hospital for helping to realize this study.
Funding
Funding for this research was provided by the Felix Mandl Fund (Scientific Fund of the City of Vienna).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethics commission of the Medical University of Vienna reviewed and approved the study protocol (no. 1223/2014). Participation was strictly voluntary. Each patient was informed about the study aims and procedures orally and in writing before informed consent was obtained.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matzka, M., Köck-Hódi, S., Jahn, P. et al. Relationship among symptom clusters, quality of life, and treatment-specific optimism in patients with cancer. Support Care Cancer 26, 2685–2693 (2018). https://doi.org/10.1007/s00520-018-4102-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4102-8