Abstract
Purpose
Even with the use of modern antiemetic drugs, chemotherapy-induced nausea and vomiting (CINV) is still a cause of great distress to the patients. Olanzapine, primarily marketed as an antipsychotic, was found to reduce nausea and vomiting in some chemotherapy patients. But it was never tested in Indian population with a diverse genetic background. The present study aims to evaluate the role of olanzapine in CINV in patients receiving platinum-based chemotherapy.
Methods
The study was a randomized, controlled, assessor-blinded study on 100 chemotherapy-naïve consenting patients receiving any one from cisplatin, carboplatin or oxaliplatin. The control group (n = 50) received palonosetron and dexamethasone in the approved therapeutic dose from the day 1 of chemotherapy. The test group (n = 50) received additional olanzapine 10 mg/day from day 1 for five consecutive days. CINV and quality of life (QoL) were assessed.
Results
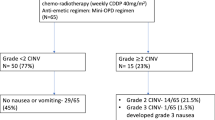
Vomiting was significantly less among the olanzapine-treated patients. Control of delayed emesis was significantly better in this group (complete response among 96 vs. 42 % in the control group, p value <0.0001). Incidence and severity of nausea was significantly less in this group. Failure of anti-CINV measure was 4 % in this group compared to 26 % of the patients of the control group during overall days 1–5. Though sedation was more in these olanzapine-treated patients, there was no dose-limiting adverse event. Quality of life was also better among the olanzapine-treated patients.
Conclusion
Olanzapine was found to be effective as add-on in the control of CINV.





Similar content being viewed by others
References
Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, et al. (1983) On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19(2):203–208
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, et al. (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7(2):189–195
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics. Cancer 97:2880–2886
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJG, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 20:107–117
Momose H, Ide T, Tateishi K, Koizumi T (2013) Incidence of chemotherapy-induced nausea and vomiting in patients receiving carboplatin-including chemotherapy. Gan To Kagaku Ryoho 40:355–359
Chasen MR, Rapoport BL (2016) Rolapitant for the treatment of chemotherapy-induced nausea and vomiting: a review of the clinical evidence. Future Oncol 12:763–778
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(suppl 5):232–243
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. (2011) Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 29(31):4189–4198
Lorusso V (2016) Management of chemotherapy-induced nausea and vomiting by risk profile: role of netupitant/palonosetron. Ther Clin Risk Manag 12:917–925
Tageja N, Groninger H (2016) Chemotherapy-induced nausea and vomiting: an overview and comparison of three consensus guidelines. Postgrad Med J 92(1083):34–40
França MS, Usón Junior PLS, Antunes YPPV, Prado BL, Donnarumma C del C, Mutão TS, et al. (2015) Assessment of adherence to the guidelines for the management of nausea and vomiting induced by chemotherapy. Einstein São Paulo Braz 13(2):221–225
Tan L, Liu J, Liu X, Chen J, Yan Z, Yang H, et al. (2009) Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res CR 28:131
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9(5):188–195
Navari RM, Einhorn LH, Loehrer PJ, Passik SD, Vinson J, McClean J, et al. (2007) A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group Study. Support Care Cancer 15(11):1285–1291
Gyawali B, Poudyal BS, Iddawela M (2016) Cheaper options in the prevention of chemotherapy-induced nausea and vomiting. J Glob Oncol 16:JGO002477
Chiu L, Chow R, Popovic M, Navari RM, Shumway NM, Chiu N, et al. (2016) Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 24(5):2381–2392
Olver IN (1992) Antiemetic study design: desirable objectives, stratifications and analyses. Br J Cancer Suppl 19:S30–S34
Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, et al. (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manag 34(2):148–159
Warr JK, Chambers CR, Cusano FL, Cuthbert CA, Mah MS (2015) Feasibility of using the Multinational Association of Supportive Care in Cancer Antiemesis Tool for assessment of chemotherapy-induced nausea and vomiting at the Tom Baker Cancer Centre. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract 21(5):348–357
Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, et al. (2008) A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16(2):201–208
Matsuda Y, Okita K, Furuhata T, Kutomi G, Yamashita K, Sato Y, et al. (2015) Evaluation of the validity of chemotherapy-induced nausea and vomiting assessment in outpatients using the Japanese version of the MASCC antiemesis tool. Support Care Cancer 23(11):3331–3339
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124
Einhorn LH, Brames MJ, Dreicer R, Nichols CR, Cullen MT, Bubalo J (2007) Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer 15(11):1293–1300
Navari RM (2009) Palonosetron for the prevention of chemotherapy-induced nausea and vomiting: approval and efficacy. Cancer Manag Res 1:167–176
Babu G, Saldanha SC, Kuntegowdanahalli Chinnagiriyappa L, Jacob LA, Mallekavu SB, Dasappa L, et al. (2016) The efficacy, safety, and cost benefit of olanzapine versus aprepitant in highly emetogenic chemotherapy: a pilot study from South India. Chemother Res Pract. doi:10.1155/2016/3439707
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358(23):2482–2494
Wang X, Feng Y, Chen Y, Gao BL, Han B (2014) A meta-analysis of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. Sci Rep 4:4813
Herrstedt J, Dombernowsky P (2007) Anti-emetic therapy in cancer chemotherapy: current status. Basic Clin Pharmacol Toxicol 101(3):143–150
Bymaster FP, Falcone JF, Bauzon D, Kennedy JS, Schenck K, DeLapp NW, et al. (2001) Potent antagonism of 5-HT(3) and 5-HT(6) receptors by olanzapine. Eur J Pharmacol 430(2–3):341–349
Kennedy JS, Bymaster FP, Schuh L, Calligaro DO, Nomikos G, Felder CC, et al. (2001) A current review of olanzapine’s safety in the geriatric patient: from pre-clinical pharmacology to clinical data. Int J Geriatr Psychiatry 16(Suppl 1):S33–S61
Djerada Z, Brousse G, Niel P, Llorca P-M, Eschalier A, Bentue-Ferrer D, et al. (2015) Therapeutic drug monitoring of olanzapine. Therapie. doi:10.2515/therapie/2015040
Rao ML, Hiemke C, Grasmäder K, Baumann P, TDM Arbeitsgruppe Der AGNP (2001) Olanzapine: pharmacology, pharmacokinetics and therapeutic drug monitoring. Fortschr Neurol Psychiatr 69(11):510–517
Brafford MV, Glode A (2014) Olanzapine: an antiemetic option for chemotherapy-induced nausea and vomiting. J Adv Pract Oncol 5(1):24–29
Mizukami N, Yamauchi M, Koike K, Watanabe A, Ichihara K, Masumori N, et al. (2014) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manag 47(3):542–550
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375(2):134–142
Brown CS, Markowitz JS, Moore TR, Parker NG (1999) Atypical antipsychotics: part II: adverse effects, drug interactions, and costs. Ann Pharmacother 33(2):210–217
Citrome L, Holt RIG, Walker DJ, Hoffmann VP (2011) Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig 31(7):455–482
Steiger H (2004) Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci JPN 29(1):20–29
Haleem DJ (2012) Serotonin neurotransmission in anorexia nervosa. Behav Pharmacol 23(5–6):478–495
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94(7):1011–1015
Liu J, Tan L, Zhang H, Li H, Liu X, Yan Z, et al. (2015) QoL evaluation of olanzapine for chemotherapy-induced nausea and vomiting comparing with 5-HT3 receptor antagonist. Eur J Cancer Care (Engl) 24(3):436–443
Acknowledgments
The authors would like to thank Dr. Basabdatta Samanta, Department of Biochemistry; Dr. Rajesh Issac and Ms. Paramdeep Kaur of the Department of Community Medicine; Dr. Jaineet Sachdeva, Dr. Preeti Negi, Dr. Sapna Marcus, Dr. Abdidha Malik, Dr. Deepti AP, Dr. Parneet Singh, Dr. Jaspinder Kaur, Dr. Babusa Kalra, Dr. Romi Grover, Ms. Rashmeet Sond, Ms. Geetanjali Dutta, Ms. Nirmal, Mr. Johny and Mr. Rajesh of the Department of Radiotherapy and Day Care Center; Dr. Kunal Jain of the Department of Medical Oncology; Ms. Pritty Titus, statistician, of the Department of Neurology, Christian Medical College & Hospital, Ludhiana.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Financial disclosure
The authors have no financial disclosure, and the study was not a sponsored study.
Rights and permissions
About this article
Cite this article
Mukhopadhyay, S., Kwatra, G., Alice K, P. et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 25, 145–154 (2017). https://doi.org/10.1007/s00520-016-3386-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3386-9