Abstract
Purpose
This multicenter phase II trial assessed the clinical benefit of a multidisciplinary oral care program in reducing the incidence of severe chemoradiotherapy-induced oral mucositis (OM).
Methods
Patients with head and neck cancer (HNC) who were scheduled to receive definitive or postoperative chemoradiotherapy were enrolled. The oral care program included routine oral screening by dentists and a leaflet containing instructions regarding oral care, nutrition, and lifestyle. Oral hygiene and oral care were evaluated continuously during and after the course of chemoradiotherapy. The primary endpoint was the incidence of grade ≥3 OM assessed by certified medical staff according to the Common Terminology Criteria of Adverse Events version 3.0.
Results
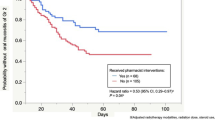
From April 2012 to December 2013, 120 patients with HNC were enrolled. Sixty-four patients (53.3 %) developed grade ≥3 OM (i.e., functional/symptomatic). The incidence of grade ≤1 OM at 2 and 4 weeks after radiotherapy completion was 34.2 and 67.6 %, respectively. Clinical examination revealed that 51 patients (42.5 %) developed grade ≥3 OM during chemoradiotherapy. The incidence of grade ≤1 OM at 2 and 4 weeks after radiotherapy completion was 54.7 and 89.2 %, respectively. The incidences of grade 3 infection and pneumonitis throughout chemoradiotherapy were <5 %. Only 6.7 % of patients had unplanned breaks in radiotherapy, and 99.2 % completed treatment.
Conclusions
A systematic oral care program alone is insufficient to decrease the incidence of severe OM in patients with HNC being treated with chemoradiotherapy. However, systematic oral care programs may indirectly improve treatment compliance by decreasing infection risk.
Trial registration number: UMIN000006660

Similar content being viewed by others
References
Marur S, Forastiere AA (2008) Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 83:489–501
Forastiere AA, Goepfert H, Maor M, et al. (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Adelstein DJ, Li Y, Adams GL, et al. (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Vera-Llonch M, Oster G, Hagiwara M, Sonis S (2006) Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 106:329–336
Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68:1110–1120
Hansen O, Overgaard J, Hansen HS, Overgaard M, Höyer M, Jörgensen KE (1997) Importance of overall treatment time for the outcome of radiotherapy of advanced head and neck carcinoma: dependency on tumor differentiation. Radiother Oncol 43:47–51
Russo G, Haddad R, Posner M, Machtay M (2008) Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 13:886–898
Bese NS, Hendry J, Jeremic B (2007) Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 68:654–661
Larson PJ, Miaskowski C, MacPhail L, et al. (1998) The PRO-SELF Mouth Aware program: an effective approach for reducing chemotherapy-induced mucositis. Cancer Nurs 21:263–268
Cheng KK, Molassiotis A, Chang AM, Wai WC, Cheung SS (2001) Evaluation of an oral care protocol intervention in the prevention of chemotherapy-induced oral mucositis in paediatric cancer patients. Eur J Cancer 37:2056–2063
Saito H, Watanabe Y, Sato K, et al. (2014) Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer 22:2935–2940
List MA, Ritter-Sterr C, Lansky SB (1990) A performance status scale for head and neck cancer patients. Cancer 66:564–569
Zenda S, Matsuura K, Tachibana H, et al. (2011) Multicenter phase II study of an opioid-based pain control program for head and neck cancer patients receiving chemoradiotherapy. Radiother Oncol 101:410–414
Bensinger W, Schubert M, Ang KK, et al. (2008) NCCN Task Force report: prevention and management of mucositis in cancer care. J Natl Compr Cancer Netw 6(Suppl 1):S1–S21
Rosenthal DI, Trotti A (2009) Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol 19:29–34
Garg S, Yoo J, Winquist E (2010) Nutritional support for head and neck cancer patients receiving radiotherapy: a systematic review. Support Care Cancer 18:667–677
Paccagnella A, Morello M, Da Mosto MC, et al. (2010) Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer 18:837–845
RV L, MC L, CH H, Ariyawardana A, D'Amato-Palumbo S, Fischer DJ, Martof A, Nicolatou-Galitis O, Patton LL, Elting LS, Spijkervet FK, Brennan MT (2010) Fungal Infections Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 18:985–992
Ishiki H, Onozawa Y, Kojima T, et al. (2012) Nutrition support for head and neck squamous cell carcinoma patients treated with chemoradiotherapy: how often and how long? ISRN Oncol 2012:274739
Sciubba JJ, Goldenberg D (2006) Oral complications of radiotherapy. Lancet Oncol 7:175–183
Reuther T, Schuster T, Mende U, Kübler A (2003) Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—a report of a thirty year retrospective review. Int J Oral Maxillofac Surg 32:289–295
Murray CG, Daly TE, Zimmerman SO (1980) The relationship between dental disease and radiation necrosis of the mandible. Oral Surg Oral Med Oral Pathol 49:99–104
Marx RE, Johnson RP (1987) Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol 64:379–390
Acknowledgments
The authors thank Drs. Takeshi Kodaira, Yusuke Onozawa, Chiyoko Makita, Tomomi Hikosaka, Yukihiko Oshima, and Akiko Todaka for patient enrollment and Mr. Akihiro Sudo for oral care equipment management. This study was supported by a grant from the National Cancer Center Research and Development Fund (23-A-30) and by Sunstar Inc. Equipment for oral care was supplied by Sunstar Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tomoya Yokota serves in an advisory role for AstraZeneca, Merck Serono, and Bristol-Myers Squibb, and has received lecture fees from Merck Serono. Toru Eguchi is an employee of Sunstar Inc. Sunstar Inc. had no control over the interpretation, writing, or publication of this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yokota, T., Tachibana, H., Konishi, T. et al. Multicenter phase II study of an oral care program for patients with head and neck cancer receiving chemoradiotherapy. Support Care Cancer 24, 3029–3036 (2016). https://doi.org/10.1007/s00520-016-3122-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3122-5