Abstract
Purpose
Cancer patients receiving chemotherapy will sometimes conceal their discomfort, but an excessive endurance for adverse drug reactions (ADRs) can lead to a poorer prognosis. The aim of this study was to clarify the association between ADR endurance and a preference of cancer patients for aggressive treatments.
Methods
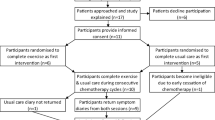
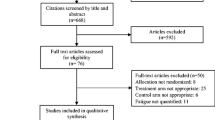
A cross-sectional study was undertaken of inpatients under 75 years of age receiving injectable systemic chemotherapy or oral chronic medications at hospitals in Japan. Subjects were asked to respond to a validated questionnaire to assess the extent of their ADR endurance and whether they would choose a novel, more aggressive therapy if their life expectancy was estimated at 2 years.
Results
Study participants were separated into the chemotherapy group (n = 36) and the non-chemotherapy group (n = 78). In the chemotherapy group, patients who had moderate ADR endurance scores were more likely to choose the new therapy (0–33, 34–67, and 68–100 points: 0.0, 54.5, and 27.3 %; χ 2 test, p = 0.15). Additionally, every patient on long-term chemotherapy (≥3 years) had high ADR endurance scores but did not choose the new, riskier treatment. In the non-chemotherapy group, the proportion of those choosing the new therapy was linearly associated with higher ADR endurance scores (25.9, 38.2, and 64.7 %; p = 0.04).
Conclusion
Cancer patients may prefer aggressive therapies, even when self-estimations of ADR endurance are not very high, especially if they have been receiving chemotherapy for a short period of time. These patients should be observed with great caution.


Similar content being viewed by others
References
Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP (2009) Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer 17:117–127. doi:10.1007/s00520-008-0505-2
Jansen SJ, Otten W, Stiggelbout AM (2004) Review of determinants of patients’ preferences for adjuvant therapy in cancer. J Clin Oncol 22:3181–3190. doi:10.1200/JCO.2004.06.109
Simes RJ, Coates AS (2001) Patient preferences for adjuvant chemotherapy of early breast cancer: how much benefit is needed? J Natl Cancer Inst Monogr 30:146–152
Jansen SJ, Kievit J, Nooij MA, de Haes JC, Overpelt IM, van Slooten H, Maartense E, Stiggelbout AM (2001) Patients’ preferences for adjuvant chemotherapy in early-stage breast cancer: is treatment worthwhile? Br J Cancer 84:1577–1585. doi:10.1054/bjoc.2001.1836
Zimmermann C, Baldo C, Molino A (2000) Framing of outcome and probability of recurrence: breast cancer patients’ choice of adjuvant chemotherapy (ACT) in hypothetical patient scenarios. Breast Cancer Res Treat 60:9–14
Ravdin PM, Siminoff IA, Harvey JA (1998) Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol 16:515–521
Duric V, Stockler M (2001) Patients’ preferences for adjuvant chemotherapy in early breast cancer: a review of what makes it worthwhile. Lancet Oncol 2:691–697. doi:10.1016/S1470-2045(01)00559-9
Slevin ML, Stubbs L, Plant HJ, Wilson P, Gregory WM, Armes PJ, Downer SM (1990) Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ 300:1458–1460
Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, Dent SF, Song X, Segal R, Mazzarello S, Crawley F, Dranitsaris G, Clemons M (2013) Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 142:101–107. doi:10.1007/s10549-013-2727-3
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13:219–227. doi:10.1007/s00520-004-0710-6
Beusterien K, Grinspan J, Kuchuk I, Mazzarello S, Dent S, Gertler S, Bouganim N, Vandermeer L, Clemons M (2014) Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 19:127–134. doi:10.1634/theoncologist.2013-0359
Sahm S, Will R, Hommel G (2005) What are cancer patients’ preferences about treatment at the end of life, and who should start talking about it? A comparison with healthy people and medical staff. Support Care Cancer 13:206–214. doi:10.1007/s00520-004-0725-z
Donovan KA, Greene PG, Shuster JL, Partridge EE, Tucker DC (2002) Treatment preferences in recurrent ovarian cancer. Gynecol Oncol 86:200–211
Lindley C, Vasa S, Sawyer WT, Winer EP (1998) Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol 16:1380–1387
Oken M, Creech R, Tormey D, Horton J, Davis T, McFadden E, Carbone P (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Iihara N, Nishio T, Okura M, Anzai H, Kagawa M, Houchi H, Kirino Y (2014) Comparing patient dissatisfaction and rational judgment in intentional medication non-adherence versus unintentional non-adherence. J Clin Pharm Ther 39:45–52. doi:10.1111/jcpt.12100
Fayers P, Machin D (2000) Quality of life: assessment, analysis and interpretation. Wiley, Chichester
Stiggelbout AM, de Haes JC (2001) Patient preference for cancer therapy: an overview of measurement approaches. J Clin Oncol 19:220–230
National Cancer Institute (2006) Common terminology criteria for adverse events. National Cancer Institute Web. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 1 Feb. 2014
Luthy C, Cedraschi C, Pugliesi A, Di Silvestro K, Mugnier-Konrad B, Rapiti E, Allaz AF (2011) Patients’ views about causes and preferences for the management of cancer-related fatigue—a case for non-congruence with the physicians? Support Care Cancer 19:363–370. doi:10.1007/s00520-010-0826-9
Martin MY, Fouad MN, Oster RA, Schrag D, Urmie J, Sanders S, Pisu M (2014) What do cancer patients worry about when making decisions about treatment? Variation across racial/ethnic groups. Support Care Cancer 22:233–244. doi:10.1007/s00520-013-1958-5
Acknowledgments
We thank Hiroshi Muguruma, Kana Sumiyoshi, Eitoku Matsuoka, Kiyo Suzuki, Yoshie Isobe, Nami Mizukawa, and Kumiko Kushida for their assistance with data collection.
Funding
This work was supported by a research grant from the Pfizer Health Research Foundation. The funding source had no role in the collection or analysis of the study data.
Conflict of interest
Dr. Houchi received a research grant from Pfizer Japan Inc. The remaining authors report no conflicts of interest. The authors have full control of all primary data and agree to allow the journal to review their data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iihara, N., Nishio, T., Goda, T. et al. Effect of endurance for adverse drug reactions on the preference for aggressive treatments in cancer patients. Support Care Cancer 23, 1091–1097 (2015). https://doi.org/10.1007/s00520-014-2439-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2439-1