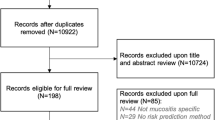
Abstract
Purpose
High-dose chemotherapy and autologous stem cell transplant (ASCT) to treat multiple myeloma (MM) and other cancers carries the risk of oral mucositis (OM) with sequelae including impaired nutritional and fluid intake, pain, and infectious complications. As a result of these problems, cancer treatment may have to be interrupted or delayed. In this study, we looked beyond OM’s known risk factors of renal function and melphalan dose with a genome-wide association study (GWAS) to evaluate whether genetic variants in conjunction with clinical risk factors influence predisposition for OM.
Methods
Genotyping was performed using Illumina HumanOmni1-Quad v1.0 BeadChip and further assessed for data quality. We tested 892,589 germline single-nucleotide polymorphisms (SNPs) for association with OM among 972 Caucasian patients treated with high-dose melphalan and ASCT in Total Therapy clinical trials (TT2, TT3, TT4) for newly diagnosed MM. Statistical analyses included t tests, stepwise regression modeling, and logistic regression modeling to find baseline clinical factors and genotypes associated with OM.
Results
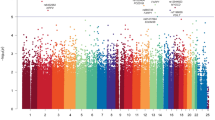
We found that 353 (36.3 %) patients had grades 2–4 OM. Type of treatment protocol, baseline estimated glomerular filtration rate, and melphalan dose along with baseline serum albumin and female gender predicted 43.6 % of grades 2–4 OM cases. Eleven SNPs located in or near matrix metalloproteinase 13, JPH3, DHRS7C, CEP192, CPEB1/LINC00692, FBN2, ALDH1A1, and DMRTA1/FLJ35282 were associated with grades 2–4 OM. The addition of these SNPs increased sensitivity in detecting grades 2–4 OM cases to 52 %.
Conclusions
These SNPs may be important for their roles in inflammatory pathways, epithelial healing, and chemotherapy detoxification.
Similar content being viewed by others
References
Barlogie B, Shaughnessy J, Tricot G et al (2004) Treatment of multiple myeloma. Blood 103(1):20–32. doi:10.1182/blood-2003-04-1045
Giralt S, Bensinger W, Goodman M et al (2003) 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood 102(7):2684–2691. doi:10.1182/blood-2002-10-3250
Blijlevens N, Schwenkglenks M, Bacon P et al (2008) Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy. European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol 26(9):1519–1525. doi:10.1200/JCO.2007.13.6028
Clarkson JE, Worthington HV, Furness S et al (2010) Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 8, CD001973. doi:10.1002/14651858.CD001973.pub4
McCann S, Schwenkglenks M, Bacon P et al (2009) The prospective oral mucositis audit: relationship of severe oral mucositis with clinical and medical resource use outcomes in patients receiving high-dose melphalan or BEAM-conditioning chemotherapy and autologous SCT. Bone Marrow Transplant 43(2):141–147. doi:10.1038/bmt.2008.299
Epstein JB (2007) Mucositis in the cancer patient and immunosuppressed host. Infect Dis Clin North Am 21(2):503–522. doi:10.1016/j.idc.2007.03.003, vii
Sharma SK, Handoo A, Choudhary D, Dhamija G, Gupta N (2013) Severe gastrointestinal mucositis following high dose melphalan therapy for multiple myeloma. World J Gastroenterol 19(5):784–785. doi:10.3748/wjg.v19.i5.784, PMCID: PMC3574610. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3574610/pdf/WJG-19-784.pdf
Murphy BA (2007) Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol 5(9 Suppl 4):13–21, http://www.oncologypractice.com/jso/journal/articles/0509s413.pdf
Tsirigotis P, Triantafyllou K, Girkas K et al (2008) Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: a video-capsule endoscopy study. Bone Marrow Transplant 42(5):337–343. doi:10.1038/bmt.2008.168
Grazziutti ML, Dong L, Miceli MH et al (2006) Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant 38(7):501–506. doi:10.1038/sj.bmt.1705471
Barlogie B, Tricot G, Anaissie E et al (2006) Thalidomide and hematopoetic-cell transplantation for multiple myeloma. N Engl J Med 354(10):1021–1030. doi:10.1056/NEJMoa053583
Barlogie B, Anaissie E, van Rhee F et al (2007) Incorporating bortezomib into upfront treatment for multiple myeloma: early results of Total Therapy 3. Br J Haematol 138(2):176–185. doi:10.1111/j.1365-2141.2007.06639.x
Anaissie EJ et al (2010) Comparing toxicities and survival outcomes with total therapy 4 (TT4) for 70-gene (R70)-defined low-risk multiple myeloma (MM) to results obtained with Total Therapy 3 Protocols TT3A and TT3B. Blood (ASH Annu Meet Abstr) 116(21), Abstract 368, http://abstracts.hematologylibrary.org/cgi/content/abstract/116/21/368?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=368+&searchid=1&FIRSTINDEX=0&volume=116&issue=21&resourcetype=HWCIT
Mosteller RD (1987) Simplified calculation of body surface area. N Engl J Med 317(17):1098. doi:10.1056/NEJM198710223171717
Common Terminology Criteria for Adverse Events (CTCAE) version 4. http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf
Levey AS, Greene T, Kusek JW et al (2000) A simplified equation to predict glomerular filtration rate from serum creatinine [abstract]. J Am Soc Nephrol 11:A0828, 155A
Cochran WG (1954) Some methods for strengthening the common chi-squared tests. Biometrics (Int Biom Soc) 10(4):417–451. doi:10.2307/3001616
Armitage P (1955) Tests for linear trends in proportions and frequencies. Biometrics (Int Biom Soc) 11(3):375–386. doi:10.2307/3001775
Benjamimi Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR Stat Soc B 57(1):289–300, http://www.jstor.org/stable/2346101
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482(7385):339–346. doi:10.1038/nature10887
Chen G, Qiu C, Zhang Q et al (2013) Genome-wide analysis of human SNPs at long intergenic noncoding RNAs. Hum Mutat 34(2):338–344. doi:10.1002/humu.22239
Sonis ST, Antin JH, Tedaldi MW, Alterovitz G (2013) SNP-based Bayesian networks can predict oral mucositis risk in autologous stem cell transplant recipients. Oral Dis 19(7):721–727. doi:10.1111/odi.12146
Sonis ST (2011) Oral mucositis. Anti Cancer Drugs 22(7):607–612. doi:10.1097/CAD.0b013e3283462086
Haynes SL, Shuttleworth CA, Kielty CM (1997) Keratinocytes express fibrillin and assemble microfibrils: implications for dermal matrix organization. Br J Dermatol 137(1):17–23. doi:10.1046/j.1365-2133.1997.1762185.x
Davis MR, Summers KM (2012) Structure and function of the mammalian fibrillin gene family: Implications for human connective tissue diseases. Mol Genet Metab 107(4):635–647. doi:10.1016/j.ymgme.2012.07.023
Nistala H, Lee-Arteaga S, Smaldone S et al (2010) Fibrillin-1 and -2 differently modulate endogenous TGF-β and BMP bioavailability during bone formation. J Cell Biol 190(6):1107–1121. doi:10.1083/jcb.201003089
Ben-Lulu S, Pollak Y, Mogilner J et al (2012) Dietary transforming growth factor-beta 2 (TGF-β2) supplementation reduces methotrexate-induced intestinal mucosal injury in a rat. PLoS One 7(9):e45221. doi:10.1371/journal.pone.0045221
de Koning BAE, Philipsen-Geerling B, Hoijer M et al (2007) Protection against chemotherapy induced mucositis by TGF-β2 in childhood cancer patients: results from a randomized cross-over study. Pediatr Blood Cancer 48(5):532–539. doi:10.1002/pbc.20910
Foncuberta MC, Cagnoni PJ, Brandts CH et al (2001) Topical transforming growth factor TGF-β3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas or solid tumors. J Immunother 24(4):384–388. doi:10.1097/00002371-200107000-00014
Bian HG, Li LF et al (2013) Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med 19(4):421–428. doi:10.1038/nm.3118
Uitto VJ, Airola K, Vaalamo M et al (1998) Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol 152(6):1489–1499, PMC1858431
Hattori N, Mochizuki S, Kishi K et al (2009) MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 175(2):533–546. doi:10.2353/ajpath.2009.081080
Toriseva M, Laato M, Carpen O et al (2012) MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS One 7(8):e42596. doi:10.1371/journal.pone.0042596
Pinto N, Ludeman SM, Dolan ME (2009) Drug focus: pharmacogenetic studies related to cyclophosphamide-based therapy. Pharmacogenomics 10(12):1897–1903. doi:10.2217/pgs.09.134
Hassan M, Andersson BS (2013) Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics 14(1):75–87. doi:10.2217/pgs.12.185
Lu B, Tigchelaar W, Ruifrok W et al (2012) DHRS7c, a novel cardiomyocyte-expressed gene that is down-regulated by adrenergic stimulation and in heart failure. Eur J Heart Fail 14(1):5–13. doi:10.1093/eurjhf/hfr152
Pearson TA, Manolio TA (2008) How to interpret a genome-wide association study. JAMA 299(11):1335–1344. doi:10.1001/jama.299.11.1335
Conflict of interest
The authors have no conflict of interest to report. Author/COI Disclosure Forms were submitted along with the manuscript.
Author contributions
EJ Anaissie, EA Coleman, JY Lee, JA Goodwin, CA Enderlin, and VR Raj are responsible for the conception and design; N Sanathkumar, EA Coleman, EJ Anaissie, and JA Goodwin for the collection and verification of clinical data; VR Raj, O Stephens, and SW Erickson for the generation and verification of GWAS data; JY Lee, SW Erickson, EA Coleman, D Zhou, VR Raj, JA Goodwin, and N Sanathkumar for data analysis and interpretation; PJ Reed for the administrative support; KD McKelvey for serving as a consultant on medical genetics; EA Coleman, JY Lee, D Zhou, SW Erickson, JA Goodwin, VR Raj, N Sanathkumar, S Apewokin, and AJ Vangsted for writing the manuscript; and EA Coleman, JY Lee, SW Erickson, JA Goodwin, N Sanathkumar, D Zhou, KD McKelvey, VR Raj, S Apewokin, O Stephens, CA Enderlin, AJ Vangsted, PJ Reed, and EJ Anaissie fo the final approval of the manuscript.
Financial support
Primary support was given by the National Institutes of Health (NIH)/National Institute of Nursing Research (NINR) 5 RC2NR011945, and for the additional support, the Translational Research Institute at UAMS (grant #1UL1RR029884) and the Elizabeth Stanley Cooper Chair in Oncology Nursing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coleman, E.A., Lee, J.Y., Erickson, S.W. et al. GWAS of 972 autologous stem cell recipients with multiple myeloma identifies 11 genetic variants associated with chemotherapy-induced oral mucositis. Support Care Cancer 23, 841–849 (2015). https://doi.org/10.1007/s00520-014-2406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2406-x