Abstract
Purpose
Prevention of chemotherapy-induced nausea and vomiting (CINV) is crucial for maintaining the quality of life of cancer patients. Female patients have been underrepresented in previous clinical studies of aprepitant or palonosetron. We performed a prospective multicenter study to investigate the efficacy and safety of triple therapy comprising these two agents and dexamethasone in female cancer patients receiving chemotherapy that included cisplatin (≥50 mg/m2).
Methods
Aprepitant was administered at a dose of 125 mg before chemotherapy on day 1 and at 80 mg on days 2 and 3. Palonosetron (0.75 mg) was given before chemotherapy on day 1. Dexamethasone was administered at a dose of 9.9 mg before chemotherapy on day 1 and at 6.6 mg on days 2–4. The primary endpoint was the the proportion of patients with a complete response (CR no vomiting and no use of rescue medication) throughout the overall period (0–120 h post-chemotherapy).
Results
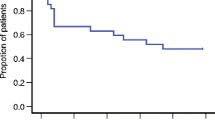
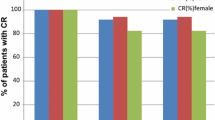
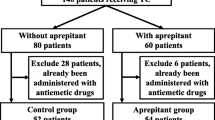
Ninety-six women (median age 55 years) were enrolled. The overall CR rate was 54.2 %. CR was obtained during the acute phase (0–24 h post-chemotherapy) and the delayed phase (24–120 h post-chemotherapy) in 87.5 and 56.3 % of the patients, respectively. The most common adverse reactions were constipation and fatigue (reported by three patients each).
Conclusions
Exhibition of a favorable overall CR rate over existing two-drug combinations suggests that the triple therapy regimen used in the present study is effective and tolerable in patients with gynecological malignancies receiving cisplatin-based chemotherapy. Female patients may have a higher risk of developing CINV.


Similar content being viewed by others
References
Richardson JL, Marks G, Levine A (1988) The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol 6:1746–1752
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. Clin Oncol 24:4472–4478
Huskey SE, Dean BJ, Bakhtiar R, Sanchez RI, Tattersall FD, Rycroft W, Hargreaves R, Watt AP, Chicchi GG, Keohane C, Hora DF, Chiu SH (2003) Brain penetration of aprepitant, a substance P receptor antagonist, in ferrets. Drug Metab Dispos 31(6):785–791
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ, Aprepitant Protocol 052 Study Group (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Grou. J Clin Oncol 21(22):4112–4119
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F, Aprepitant Protocol 054 Study Group (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97(12):3090–3098
Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Bohidar N, Klinger G, Hustad CM, Horgan KJ, Skobieranda F (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23(12):2822–2830
Chawla SP, Grunberg SM, Gralla RJ, Hesketh PJ, Rittenberg C, Elmer ME, Schmidt C, Taylor A, Carides AD, Evans JK, Horgan KJ (2003) Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 97(9):2290–2300
Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K (2011) Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 11:2455–2461
Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L, Parnes H, Whiting RL, Eglen RM (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114(4):851–859
Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, Rubenstein E, Sebastiani S, Cantoreggi S, Snyder SH, Slusher B (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107(2):469–478
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29(31):4189–4198
MASCC/ESMO Antiemetic Guideline 2013. http://www.mascc.org/assets/documents/mascc_guidelines_english_2013.pdf Accessed 8 Aug 2013
National Comprehensive Cancer Network (2014) NCCN Clinical Practice Guidelines in Oncology: Antiemesis, Version 1. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf Accessed 8 Aug 2013
Japan Society of Clinical Oncology (2010) Guidelines for the Proper Use of Antiemetics, Version 1. Kanehara, Tokyo
Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, von Pawel J, Giezek H, Ahmed T, Chan CY (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17:1000–1006
Hesketh PJ, Aapro M, Street JC, Carides AD (2010) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171–1177
Longo F, Mansueto G, Lapadula V, De Sanctis R, Quadrini S, Grande R, Gori B, Altavilla A, D’Antoni I, Del Signore E, Stumbo L, De Luca C, Cimadon B, Cortesi E, Gamucci T, Di Seri M (2011) Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 19:1159–1164
Miura S, Watanabe S, Sato K, Makino M, Kobayashi O, Miyao H, Iwashima A, Okajima M, Tanaka J, Tanaka H, Kagamu H, Yokoyama A, Narita I, Yoshizawa H (2013) The efficacy of triplet antiemetic therapy with 0.75 mg of palonosetron for chemotherapy-induced nausea and vomiting in lung cancer patients receiving highly emetogenic chemotherapy. Support Care Cancer 21(9):2575–2581
Hashimoto H, Yamanaka T, Shimada Y, Arata K, Matsui R, Goto K, Takiguchi T, Ohyanagi F, Kogure Y, Nogami N, Nakao M, Takeda K, Azuma K, Nagase S, Hayashi T, Fujiwara K, Shimada T, Seki N, Suzuki K, Yamamoto N (2013) Palonosetron (PALO) versus granisetron (GRA) in the triplet regimen with dexamethasone (DEX) and aprepitant (APR) for preventing chemotherapy-induced nausea and vomiting (CINV) in patients (pts) receiving highly emetogenic chemotherapy (HEC) with cisplatin (CDDP): A randomized, double-blind, phase III trial. ASCO2013 Annual Meeting abstr #9621 http://meetinglibrary.asco.org/content/111042-132 Accessed 8 Aug 2013
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9(5):188–195
Acknowledgments
We would like to thank Dr. Noriyuki Takai for his substantial support with the conception and implementation of the present study.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeshima, N., Matoda, M., Abe, M. et al. Efficacy and safety of triple therapy with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy for gynecological cancer: KCOG-G1003 phase II trial. Support Care Cancer 22, 2891–2898 (2014). https://doi.org/10.1007/s00520-014-2280-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2280-6