Abstract
Purpose
This study aimed to determine the antiemetic efficacy and safety of a combination of palonosetron, aprepitant, and dexamethasone in patients with testicular germ cell tumor (TGCT) receiving 5-day cisplatin-based combination chemotherapy.
Methods
An open-label, single-arm, multicenter study was performed in patients with TGCT who were scheduled to receive 5-day cisplatin-based combination chemotherapy. The antiemetic therapy consisted of palonosetron 0.75 mg on day 1, aprepitant 125 mg on day 1 and 80 mg on days 2 to 5, and dexamethasone 9.9 mg on day 1 and 6.6 mg on days 2 to 8. The primary endpoint was complete response (CR) rate, which was defined as no vomiting and no rescue medication, in the overall period (0 to 216 h) in the first chemotherapy course. Incidence and severity of nausea were assessed based on the Common Terminology Criteria for Adverse Events (CTCAE) and a subjective rating scale completed by patients.
Results
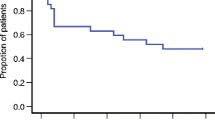
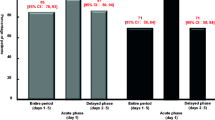
Thirty patients were included in the analysis. CR was achieved in 90.0 % of the patients in the first chemotherapy course, and high CR rates were also observed in the second and third courses (82.1 and 78.3 %, respectively). The incidence of nausea peaked on days 4 to 6 in about 50 % of the patients. The reported adverse drug reactions were hiccups (13.3 %), anorexia (3.3 %), and stomach pain (3.3 %). None of these were unexpected and none were grade 3 or 4.
Conclusions
The combination antiemetic therapy examined in this study was highly effective and well-tolerated in patients with TGCT receiving 5-day cisplatin-based combination chemotherapy.


Similar content being viewed by others
References
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13:219–227
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Tavorath R, Hesketh PJ (1996) Drug treatment of chemotherapy-induced delayed emesis. Drugs 52:639–648
Kondagunta GV, Motzer RJ (2006) Chemotherapy for advanced germ cell tumors. J Clin Oncol 24:5493–5502
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, ESMO/MASCC Guidelines Working Group (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243
Einhorn LH, Rapoport B, Koeller J, Grunberg SM, Feyer P, Rittenberg C, Aapro M (2005) Antiemetic therapy for multiple-day chemotherapy and high-dose chemotherapy with stem cell transplant: review and consensus statement. Support Care Cancer 13:112–116
Oo TH, Hesketh PJ (2005) Drug insight: new antiemetics in the management of chemotherapy-induced nausea and vomiting. Nat Clin Pract Oncol 2:196–201
Einhorn LH, Brames MJ, Dreicer R, Nichols CR, Cullen MT Jr, Bubalo J (2007) Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer 15:1293–1300
Jordan K, Kinitz I, Voigt W, Behlendorf T, Wolf HH, Schmoll HJ (2009) Safety and efficacy of a triple antiemetic combination with the NK-1 antagonist aprepitant in highly and moderately emetogenic multiple-day chemotherapy. Eur J Cancer 45:1184–1187
Ajioka H, Morita F, Akizawa Y, Yoshida K, Kitamura R, Takimoto H (2010) Pharmacological, pharmacokinetic, and clinical profile of palonosetron hydrochloride (ALOXI I.V. injection 0.75 mg), a novel antiemetic 5-HT3-receptor antagonist. Nihon Yakurigaku Zasshi 136:113–120 (in Japanese)
Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, Kindler H, Grote TH, Pendergrass K, Grunberg SM, Carides AD, Gertz BJ (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med 340:190–195
National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 24 November 2013
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94:1011–1015
Cook AM, Dzik-Jurasz AS, Padhani AR, Norman A, Huddart RA (2001) The prevalence of avascular necrosis in patients treated with chemotherapy for testicular tumours. Br J Cancer 85:1624–1626
van den Berkmortel F, de Wit R, de Rooy J, DeMulder P (2004) Osteonecrosis in patients with testicular tumours treated with chemotherapy. Neth J Med 62:23–27
Albany C, Brames MJ, Fausel C, Johnson CS, Picus J, Einhorn LH (2012) Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a Hoosier Oncology Group study. J Clin Oncol 30:3998–4003
Multinational Association of Supportive Care in Cancer. MASCC/ESMO Antiemetic Guideline 2013. http://www.mascc.org/assets/documents/mascc_guidelines_english_2013.pdf. Accessed 24 November 2013
Olver IN, Grimison P, Chatfield M, Stockler MR, Toner GC, Gebski V, Harrup R, Underhill C, Kichenadasse G, Singhal N, Davis ID, Boland A, McDonald A, Thomson D, Australian and New Zealand Urogenital and Prostate Cancer Trials Group (2013) Results of a 7-day aprepitant schedule for the prevention of nausea and vomiting in 5-day cisplatin-based germ cell tumor chemotherapy. Support Care Cancer 21:1561–1568
Matsuda T, Saika K (2008) Comparison of time trends in testicular cancer incidence (1973–97) in East Asia, Europe and USA, from cancer incidence in five continents. Vols IV–VIII. Jpn J Clin Oncol 38:578–579
Conflict of interest
Shota Hamada was an employee of MSD K.K., a subsidiary of Merck & Co., Inc. Whitehouse Station, NJ, USA, when the study was conducted. Hiroyuki Nishiyama received honoraria, grants, or other funding from Taiho Pharmaceutical Co., Ltd. and received grants or other funding from Ono Pharmaceutical Co., Ltd. Tomonori Habuchi, Osamu Ogawa, and Koji Kawakami received grants and other funding from Taiho Pharmaceutical Co., Ltd. The other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13.9 KB)
Rights and permissions
About this article
Cite this article
Hamada, S., Hinotsu, S., Kawai, K. et al. Antiemetic efficacy and safety of a combination of palonosetron, aprepitant, and dexamethasone in patients with testicular germ cell tumor receiving 5-day cisplatin-based combination chemotherapy. Support Care Cancer 22, 2161–2166 (2014). https://doi.org/10.1007/s00520-014-2182-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2182-7