Abstract
Purpose
Adherence to guideline-consistent chemotherapy-induced nausea and vomiting (CINV) prophylaxis is suboptimal. The primary aim of this study was to evaluate the magnitude of compliance to institutional guideline-directed antiemetic prophylaxis using a computerized physician order entry system at a single tertiary care institution. A nurse survey was also performed to evaluate how oncology practices, within a cooperative group, managed clinician orders for the prevention of CINV.
Methods
The electronic medical records of 100 consecutive patients were evaluated. The primary endpoint was the incidence of compliance to provide all aspects of scheduled institutional guideline-directed antiemetic prophylaxis for acute (day 1) and delayed (days 2–4) CINV. A descriptive analysis was performed on the convenience sample. Logistic regression was completed to determine the predictors of noncompliance.
Results
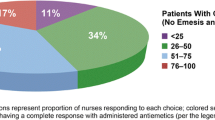
The incidence of compliance on days 1–4 was 94 %. Half of the noncompliant events (three of six, 50 %) occurred on day 1 alone and involved patients receiving low-emetogenic chemotherapy. There was a high degree of compliance to institutional guidelines for the treatment of delayed CINV (97 %). Patients receiving minimally emetogenic and moderately emetogenic chemotherapy (N = 70) were observed to be 100 % compliant. Patients receiving doxorubicin/cyclophosphamide were numerically less likely to receive institutional guidelines, compared to patients receiving other chemotherapy regimens (OR, 0.24 (0.04, 1.36), p value, 0.05). The nurse survey suggested significant variability amongst the involved institutions with regards to antiemetic prescribing practices.
Conclusions
Computerized physician order entry is associated with impressive adherence to clinician-prescribing practices, according to institutional guidelines, for acute and delayed CINV.

Similar content being viewed by others
References
NCCN (2012) Antiemesis. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 19 May 2012, Version 1. 2012
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol 29(31):4189–4198. doi:10.1200/JCO.2010.34.4614
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol Off J Eur Soc Med Oncol ESMO 21(Suppl 5):v232–v243. doi:10.1093/annonc/mdq194
Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suarez C, Zorrilla I, Gomez J, Zabaleta P, Nocea G, Llombart-Cussac A (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20(12):3141–3148. doi:10.1007/s00520-012-1448-1
Drug Utilization Review Team in Oncology (DURTO) (2003) Antiemetic prescription in Italian breast cancer patients submitted to adjuvant chemotherapy. Support Care Cancer 11(12):785–789. doi:10.1007/s00520-003-0478-0
Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol Off J Eur Soc Med Oncol ESMO. doi:10.1093/annonc/mds021
Burmeister H, Aebi S, Studer C, Fey MF, Gautschi O (2012) Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting. Support Care Cancer 20(1):141–147. doi:10.1007/s00520-010-1079-3
Fabi A, Barduagni M, Lauro S, Portalone L, Mauri M, Marinis F, Narduzzi C, Tonini G, Giampaolo M, Pacetti U, Paoloni F, Cognetti F (2003) Is delayed chemotherapy-induced emesis well managed in oncological clinical practice? An observational study. Support Care Cancer 11(3):156–161. doi:10.1007/s00520-002-0427-3
Grunberg SM (2009) Obstacles to the implementation of antiemetic guidelines. J Natl Compr Cancer Netw JNCCN 7(5):601–605
Roila F (2004) Transferring scientific evidence to oncological practice: a trial on the impact of three different implementation strategies on antiemetic prescriptions. Support Care Cancer 12(6):446–453. doi:10.1007/s00520-003-0553-6
Mertens WC, Higby DJ, Brown D, Parisi R, Fitzgerald J, Benjamin EM, Lindenauer PK (2003) Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol Off J Am Soc Clin Oncol 21(7):1373–1378
Dranitsaris G, Leung P, Warr D (2001) Implementing evidence based antiemetic guidelines in the oncology setting: results of a 4-month prospective intervention study. Support Care Cancer 9(8):611–618
Nolte MJ, Berkery R, Pizzo B, Baltzer L, Grossano D, Lucarelli CD, Kris MG (1998) Assuring the optimal use of serotonin antagonist antiemetics: the process for development and implementation of institutional antiemetic guidelines at Memorial Sloan-Kettering Cancer Center. J Clin Oncol Off J Am Soc Clin Oncol 16(2):771–778
Loprinzi CL, Alberts SR, Christensen BJ, Hanson LJ, Farley DR, Broers JK, Betcher DL, Grady RE, Southorn PA, Johnson TM, Perez EA (2000) History of the development of antiemetic guidelines at Mayo Clinic Rochester. Mayo Clinic Proc Mayo Clinic 75(3):303–309. doi:10.4065/75.3.303
Chen AR, Lehmann CU (2011) Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. J Oncol Pract Am Soc Clin Oncol 7(4):218–222. doi:10.1200/JOP.2011.000344
Harshberger CA, Harper AJ, Carro GW, Spath WE, Hui WC, Lawton JM, Brockstein BE (2011) Outcomes of computerized physician order entry in an electronic health record after implementation in an outpatient oncology setting. J Oncol Pract Am Soc Clin Oncol 7(4):233–237. doi:10.1200/JOP.2011.000261
Sklarin NT, Granovsky S, O'Reilly EM, Zelenetz AD (2011) Electronic chemotherapy order entry: a major cancer center's implementation. J Oncol Pract Am Soc Clin Oncol 7(4):213–218. doi:10.1200/JOP.2011.000266
Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B (2011) Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 19(6):823–832. doi:10.1007/s00520-010-0908-8
Celio L, Denaro A, Agustoni F, Bajetta E (2012) Palonosetron plus 1-day dexamethasone for the prevention of nausea and vomiting due to moderately emetogenic chemotherapy: effect of established risk factors on treatment outcome in a phase III trial. J Support Oncol 10(2):65–71. doi:10.1016/j.suponc.2011.06.007
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124. doi:10.1016/S1470-2045(08)70313-9
De Angelis V, Roila F, Patoia L, Lopez M, Cetto G.L, Sabbatini R, Carreca I, Manente P, Del Favero A (2000) Impact on antiemetic prescriptions of the Consensus Conference (CC) and of an expert’s visit to oncological centers. Proc Am Soc Clin Oncol 19: 606a, 2000 (abstr 2386)
Italian Group for Antiemetic Research (1998) Transferability to clinical practice of the results of controlled clinical trials: the case of antiemetic prophylactic treatment for cancer chemotherapy-induced nausea and vomiting. Ann Oncol Off J Eur Soc Med Oncol ESMO 9(7):759–765
Italian Group for Antiemetic Research (2004). Cancer patients submitted to innovative chemotherapeutic agents of intermediate emetogenic potential: antiemetic prescriptions and incidence of emesis. Tumori, 90: 103-106. doi:10.1700/210.2345
Glaus A, Knipping C, Morant R, Bohme C, Lebert B, Beldermann F, Glawogger B, Ortega PF, Husler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12(10):708–715. doi:10.1007/s00520-004-0662-x
Mertens MC, Cassells LJ, Brown DE, Koertge V, Cabana L, Parisi R, Naglieri-Prescod D, Higby DJ (2006) Chemotherapy ordering in a computerized physician order entry (CPOE) environment: a longitudinal analysis of defects from oncologist to patient. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I 24 (June 20 Supplement):18S
Molassiotis A, Brearley SG, Stamataki Z (2011) Use of antiemetics in the management of chemotherapy-related nausea and vomiting in current UK practice. Support Care Cancer 19(7):949–956. doi:10.1007/s00520-010-0909-7
Roscoe JA, Heckler CE, Morrow GR, Mohile SG, Dakhil SR, Wade JL, Kuebler JP (2012) Prevention of delayed nausea: a university of Rochester cancer center community clinical oncology program study of patients receiving chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol 30(27):3389–3395. doi:10.1200/JCO.2011.39.8123
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR (1999) Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA J Am Med Assoc 282(15):1458–1465
Author information
Authors and Affiliations
Corresponding author
Additional information
Research Grants: This work was supported by the United States National Institutes of Health Grant-CA 124477 (PI Charles Loprinzi MD).
Rights and permissions
About this article
Cite this article
Kadakia, K.C., Leal, A.D., Seisler, D.K. et al. Antiemetic prescribing practices using a computerized physician order entry system. Support Care Cancer 22, 217–223 (2014). https://doi.org/10.1007/s00520-013-1969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1969-2