Abstract
Purpose
This analysis evaluated patient-reported outcomes and analgesic use in patients with bone metastases from solid tumours across three comparative studies of denosumab and zoledronic acid.
Methods
Pooled data were analysed from three identically designed double-blind phase III studies comparing subcutaneous denosumab 120 mg with intravenous zoledronic acid 4 mg monthly in patients with bone metastases from breast cancer (n = 2,046), castration-resistant prostate cancer (n = 1,901) or other solid tumours (n = 1,597). Pain severity, pain interference, health-related quality of life and analgesic use were quantified.
Results
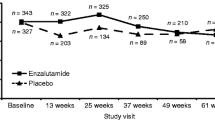
At baseline, approximately half of patients had no/mild pain (53 % [1,386/2,620] denosumab; 50 % [1,297/2,578] zoledronic acid). Denosumab delayed onset of moderate/severe pain by 1.8 months (median, 6.5 vs 4.7 months; hazard ratio, 0.83; 95 % CI, 0.76–0.92; p < 0.001; 17 % risk reduction) and clinically meaningful increases in overall pain interference by 2.6 months (median, 10.3 vs 7.7 months; hazard ratio, 0.83; 95 % CI, 0.75–0.92; p < 0.001; 17 % risk reduction) compared with zoledronic acid. Strong opioid use and worsening of health-related quality of life were less common with denosumab.
Conclusions
Across three large studies of patients with advanced solid tumours and bone metastases, denosumab prevented progression of pain severity and pain interference more effectively than zoledronic acid.




Similar content being viewed by others
References
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s
Dranitsaris G, Hatzimichael E (2012) Interpreting results from oncology clinical trials: a comparison of denosumab to zoledronic acid for the prevention of skeletal-related events in cancer patients. Support Care Cancer 20:1353–1360
van der Linden YM, Lok JJ, Steenland E, Martijn H, van Houwelingen H, Marijnen CA, Leer JW (2004) Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys 59:528–537
Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ (1994) Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330:592–596
Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19:1985–1991
Janjan N (2001) Bone metastases: approaches to management. Semin Oncol 28:28–34
National Comprehensive Cancer Network (2013) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 1.2013). National Comprehensive Cancer Network
National Comprehensive Cancer Network (2013) NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer (Version 1.2013). National Comprehensive Cancer Network
National Comprehensive Cancer Network (2013) NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer (Version 2.2013). National Comprehensive Cancer Network
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28:5132–5139
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377:813–822
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29:1125–1132
Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, Richardson GE, Siena S, Maroto P, Clemens M, Bilynskyy B, Charu V, Beuzeboc P, Rader M, Viniegra M, Saad F, Ke C, Braun A, Jun S (2012) Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 48:3082–3092
(2012) Zometa® (zoledronic acid) Injection. Full prescribing information. Novartis Pharmaceuticals Corporation
Cleeland C (2009) The brief pain inventory user guide. The University of Texas MD. Anderson Cancer Center, Houston
Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC (1996) Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain 67:267–273
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Chung KC, Barlev A, Braun A, Qian Y, Zagari M (2009) Development of the Analgesic Quantification Algorithm (AQA): a new scale to assess analgesic use [Poster]. Joint 15th Congress of the European CanCer Organisation and 34th Congress of the European Society for Medical Oncology
Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K (2011) Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol 9:72–78
Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL (2000) Defining the clinically important difference in pain outcome measures. Pain 88:287–294
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1:79
Goblirsch MJ, Zwolak PP, Clohisy DR (2006) Biology of bone cancer pain. Clin Cancer Res 12:6231s–6235s
Yoneda T, Hata K, Nakanishi M, Nagae M, Nagayama T, Wakabayashi H, Nishisho T, Sakurai T, Hiraga T (2011) Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone 48:100–105
Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O'Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW (2000) Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 6:521–528
Kaasa S, Apolone G, Klepstad P, Loge JH, Hjermstad MJ, Corli O, Strasser F, Heiskanen T, Costantini M, Zagonel V, Groenvold M, Fainsinger R, Jensen MP, Farrar JT, McQuay H, Rothrock NE, Cleary J, Deguines C, Caraceni A (2011) Expert conference on cancer pain assessment and classification—the need for international consensus: working proposals on international standards. BMJ Support Palliat Care 1:281–287
von Moos R, Strasser F, Gillessen S, Zaugg K (2008) Metastatic bone pain: treatment options with an emphasis on bisphosphonates. Support Care Cancer 16:1105–1115
Milani B, Scholten W (2011) The World Medicines Situation 2011: Access to Controlled Medicines (apps.who.int/medicinedocs/documents/s18062en/s18062en.pdf). World Health Organization (WHO), Geneva
Gater A, Abetz-Webb L, Battersby C, Parasuraman B, McIntosh S, Nathan F, Piault EC (2011) Pain in castration-resistant prostate cancer with bone metastases: a qualitative study. Health Qual Life Outcomes 9:88
Brucker PS, Yost K, Cashy J, Webster K, Cella D (2005) General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof 28:192–211
DePuy V, Anstrom KJ, Castel LD, Schulman KA, Weinfurt KP, Saad F (2007) Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 15:869–876
Saad F (2005) Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer 4:31–37
Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, Murphy R, Mason T, Cameron D (2005) Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer 92:1869–1876
Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F (2006) Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol 17:986–989
Acknowledgements
This work was supported by Amgen Inc. Jonathan Latham (whose work was funded by Amgen Inc.) and Vidya S. Beckman of Amgen Inc. provided medical writing assistance in the preparation of the manuscript.
Disclosures
The authors disclose the following potential conflicts of interest. Consultant—Amgen (RvM, JJB, AS, JEB, LJF, GM, CSC and DLP); Novartis (RvM, JJB, AS and JEB); Roche (RvM) and Bristol Myers Squibb (JEB). Remuneration—Amgen (RvM, JJB, JEB, LJF and GM); Novartis (RvM and JJB) and Roche (RvM). Funding—Amgen (RvM and JEB); Roche (RvM) and Novartis (JEB). Employment and stock ownership—Amgen (YQ, AB and KC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Portions of this work were presented at the European Oncology Nursing Society (EONS) 8th Spring Convention, 26–27 April 2012 in Geneva, Switzerland.
Trial Registration: The three studies are registered at www.clinicaltrials.gov as NCT00321464, NCT00321620 and NCT00330759.
Rights and permissions
About this article
Cite this article
von Moos, R., Body, JJ., Egerdie, B. et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer 21, 3497–3507 (2013). https://doi.org/10.1007/s00520-013-1932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1932-2