Abstract
Background
Supportive care interventions can have a substantial impact on side effects of chemotherapy. Consequently, accurate reporting of such interventions is essential when interpreting clinical trial results. This study determined the prevalence and quality of reporting of supportive care treatment for common chemotherapy-induced toxicities in phase III, breast cancer chemotherapy trials.
Methods
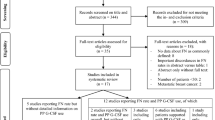
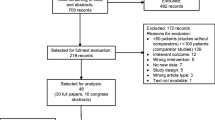
A systematic review of phase III trials of breast cancer trials incorporating chemotherapy published in the last 5 years was undertaken. Trials were identified through MEDLINE, EMBASE, BIOSIS, and the Cochrane Library. Supportive treatments evaluated were use of antiemetics, colony-stimulating growth factors, and antibiotics. Reporting quality was rated as “good”, “fair”, “poor”, or “absent” using predetermined criteria.
Results
Sixty-two trials met inclusion criteria. In 41 studies (66%), details regarding prophylactic antiemetic treatment were not provided. Growth factor use was not reported in 20 trials (32%). Instructions for the use of prophylactic antibiotics were absent in 45 trials (72%).
Conclusion
There are significant deficiencies in reporting of use of prophylactic supportive care agents in breast cancer trials. Omission of supportive care instructions may impact substantially on patient management and health care system expenditure. Recommendations for the type, dose, and frequency of supportive care drugs should be provided and reported on in trials.


Similar content being viewed by others
References
Schultz KF (1996) Randomised trials, human nature, and reporting guidelines. Lancet 348:596–598
Armitage P (1982) The role of randomization in clinical trials. Stat Med 1:345–352
Lorenz KA (2008) Progress in quality-of-care research and hope for supportive cancer care. J Clin Oncol 26:3821–3823
Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D (2002) Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 95:155–163
Mosteller F, Gilbert JP, McPeek B (1980) Reporting standards and research strategies for controlled trials: agenda for the editor. Control Clin Trials 1:37–58
Ioannidis JP, Contopoulos-Ioannidis DP (1998) Reporting of safety data from randomised trials. Lancet 352:1752–1753
Warr DG, Hesketh PJ, Gralla RJ et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830
von Minckwitz G, Kummel S, du Bois A et al (2008) Pegfilgrastim +/− ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298
Gralla RJ, Itri LM, Pisko SE et al (1981) Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med 305:905–909
Gralla RJ, Osoba D, Kris MG et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17:2971–2994
Warr DG, Grunberg SM, Gralla RJ et al (2005) The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer 41:1278–1285
Gabrilove JL, Jakubowski A, Scher H et al (1988) Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional cell carcinoma of the urothelium. N Engl J Med 318:1414–1422
Office for Human Research Protections. International Compilation of Human Research Protections 2009 Edition. US Department of Health and Human Services. Available at: http://www.hhs.gov/ohrp/international/HSPCompilation.pdf. Accessed on 18 Mar 2009.
Altman DG, Schulz KF, Moher D et al (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134:663–694
Frasci G, D'Aiuto G, Comella P et al (2006) Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a SICOG phase III study. Br J Cancer 95:1005–1012
Langley RE, Carmichael J, Jones AL et al (2005) Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclophosphamide as first-line chemotherapy for metastatic breast cancer: United Kingdom National Cancer Research Institute trial AB01. J Clin Oncol 23:8322–8330
Fountzilas G, Skarlos D, Dafni U et al (2005) Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 16:1762–1771
Fountzilas G, Kalofonos HP, Dafni U et al (2004) Paclitaxel and epirubicin versus paclitaxel and carboplatin as first-line chemotherapy in patients with advanced breast cancer: a phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 15:1517–1526
Reyno L, Seymour L, Tu D et al (2004) Phase III study of N, N-diethyl-2-[4-(phenylmethyl) phenoxy]ethanamine (BMS-217380–01) combined with doxorubicin versus doxorubicin alone in metastatic/recurrent breast cancer: National Cancer Institute of Canada Clinical Trials Group Study MA.19. J Clin Oncol 22:269–276
Pacilio C, Morabito A, Nuzzo F et al (2006) Is epirubicin effective in first-line chemotherapy of metastatic breast cancer (MBC) after an epirubicin-containing adjuvant treatment? A single centre phase III trial. Br J Cancer 94:1233–1236
Bontenbal M, Creemers GJ, Braun HJ et al (2005) Phase II to III study comparing doxorubicin and docetaxel with fluorouracil, doxorubicin, and cyclophosphamide as first-line chemotherapy in patients with metastatic breast cancer: results of a Dutch Community Setting Trial for the Clinical Trial Group of the Comprehensive Cancer Centre. J Clin Oncol 23:7081–7088
Chua S, Smith IE, A'Hern RP et al (2005) Neoadjuvant vinorelbine/epirubicin (VE) versus standard adriamycin/cyclophosphamide (AC) in operable breast cancer: analysis of response and tolerability in a randomised phase III trial (TOPIC 2). Ann Oncol 16:1435–1441
O'Brien ME, Wigler N, Inbar M et al (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15:440–449
von Minckwitz G, Raab G, Caputo A et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685
von Minckwitz G, Kummel S, Vogel P et al (2008) Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst 100:542–551
Schmid P, Untch M, Kosse V et al (2007) Leuprorelin acetate every 3 months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol 25:2509–2515
Kerbrat P, Roche H, Bonneterre J et al (2007) Epirubicin-vinorelbine vs FEC100 for node-positive, early breast cancer: French Adjuvant Study Group 09 trial. Br J Cancer 96:1633–1638
Ejlertsen B, Mouridsen HT, Jensen MB et al (2007) Improved outcome from substituting methotrexate with epirubicin: results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 43:877–884
Poole CJ, Earl HM, Hiller L et al (2006) Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Eng J Med 355:1851–1862
Gradishar WJ, Tjulandin S, Davidson N et al (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Jones SE, Erban J, Overmoyer B et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Eng J Med 352:2302–2313
Schmid P, Schippinger W, Nitsch T et al (2005) Up-front tandem high-dose chemotherapy compared with standard chemotherapy with doxorubicin and paclitaxel in metastatic breast cancer: results of a randomized trial. J Clin Oncol 23:432–440
Smith IE, A'Hern RP, Coombes GA et al (2004) A novel continuous infusional 5-fluorouracil-based chemotherapy regimen compared with conventional chemotherapy in the neo-adjuvant treatment of early breast cancer: 5 year results of the TOPIC trial. Ann Oncol 15:751–758
Martin M, Villar A, Sole-Calvo A et al (2003) Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol 14:833–842
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Eng J Med 357:2666–2676
Thomas ES, Gomez HL, Li RK et al (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210–5217
Pandya KJ, Hu P, Osborne CK et al (2007) Phase III study of standard combination versus rotating regimen of induction chemotherapy in patients with hormone insensitive metastatic breast cancer: an Eastern Cooperative Oncology Group Intergroup Study (E3185). Am J Clin Oncol 30:113–125
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Eng J Med 355:2733–2743
Levy C, Fumoleau P (2005) Gemcitabine plus docetaxel: a new treatment option for anthracycline pretreated metastatic breast cancer patients? Cancer Treat Rev 31(Suppl 4):S17–S22
Evans TR, Yellowlees A, Foster E et al (2005) Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an Anglo-Celtic Cooperative Oncology Group study. J Clin Oncol 23:2988–2995
Zielinski C, Beslija S, Mrsic-Krmpotic Z et al (2005) Gemcitabine, epirubicin, and paclitaxel versus fluorouracil, epirubicin, and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: a Central European Cooperative Oncology Group International, multicenter, prospective, randomized phase III trial. J Clin Oncol 23:1401–1408
Icli F, Akbulut H, Uner A et al (2005) Cisplatin plus oral etoposide (EoP) combination is more effective than paclitaxel in patients with advanced breast cancer pretreated with anthracyclines: a randomised phase III trial of Turkish Oncology Group. Br J Cancer 92(4):639–644
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Keller AM, Mennel RG, Georgoulias VA et al (2004) Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22:3893–3901
Ejlertsen B, Mouridsen HT, Langkjer ST et al (2004) Phase III study of intravenous vinorelbine in combination with epirubicin versus epirubicin alone in patients with advanced breast cancer: a Scandinavian Breast Group Trial (SBG9403). J Clin Oncol 22:2313–2320
Parnes HL, Cirrincione C, Aisner J et al (2003) Phase III study of cyclophosphamide, doxorubicin, and fluorouracil (CAF) plus leucovorin versus CAF for metastatic breast cancer: Cancer and Leukemia Group B 9140. J Clin Oncol 21:1819–1824
Therasse P, Mauriac L, Welnicka-Jaskiewicz M et al (2003) Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol 21:843–850
Sledge GW, Neuberg D, Bernardo P et al (2003) Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 21:588–592
Baldini E, Gardin G, Giannessi PG et al (2003) Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: a randomized phase III trial in locally advanced breast cancer. Ann Oncol 14:227–232
Bonneterre J, Roche H, Monnier A et al (2002) Docetaxel vs 5-fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. Br J Cancer 87:1210–1215
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Rivera E, Mejia JA, Arun BK et al (2008) Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer 112:1455–1461
Francis P, Crown J, Di LA et al (2008) Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst 100:121–133
Martin M, Ruiz A, Munoz M et al (2007) Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol 8:219–225
Linden HM, Haskell CM, Green SJ et al (2007) Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). J Clin Oncol 25:656–661
Jones SE, Savin MA, Holmes FA et al (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387
Harvey V, Mouridsen H, Semiglazov V et al (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24:4963–4970
Roche H, Kerbrat P, Bonneterre J et al (2006) Complete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trial. Ann Oncol 17:1221–1227
Robert N, Leyland-Jones B, Asmar L et al (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 24:2786–2792
Rouesse J, de la Lande B, Bertheault-Cvitkovic F et al (2006) A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: final results. Int J Rad Oncol 64:1072–1080
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
Alba E, Martin M, Ramos M et al (2004) Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol 22:2587–2593
Chan S, Davidson N, Juozaityte E et al (2004) Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann Oncol 15:1527–1534
Papaldo P, Lopez M, Cortesi E et al (2003) Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol 21:3462–3468
Nabholtz JM, Falkson C, Campos D et al (2003) Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 21:968–975
Conte PF, Guarneri V, Bruzzi P et al (2004) Concomitant versus sequential administration of epirubicin and paclitaxel as first-line therapy in metastatic breast carcinoma: results for the Gruppo Oncologico Nord Ovest randomized trial. Cancer 101:704–712
Martin M, Lluch A, Segui MA et al (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 17:1205–1212
Kummel S, Krocker J, Kohls A et al (2006) Randomised trial: survival benefit and safety of adjuvant dose-dense chemotherapy for node-positive breast cancer. Br J Cancer 94:1237–1244
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23:3676–3685
Tokuda Y, Tajima T, Narabayashi M et al (2008) Phase III study to evaluate the use of high-dose chemotherapy as consolidation of treatment for high-risk postoperative breast cancer: Japan Clinical Oncology Group study, JCOG 9208. Cancer Sci 99:145–151
Moore HC, Green SJ, Gralow JR et al (2007) Intensive dose-dense compared with high-dose adjuvant chemotherapy for high-risk operable breast cancer: Southwest Oncology Group/Intergroup study 9623. J Clin Oncol 25:1677–1682
Venturini M, Del ML, Aitini E et al (2005) Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 97:1724–1733
Nitz UA, Mohrmann S, Fischer J et al (2005) Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet 366:1935–1944
von Minckwitz G, Chernozemsky I, Sirakova L et al (2005) Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs 16:871–877
Feher O, Vodvarka P, Jassem J et al (2005) First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol 16:899–908
Jones SE, Holmes FA, O'Shaughnessy J et al (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27:1177–1183
Soong D, Haj R, Leung MG et al (2009) High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol 27(26):e101–e102
Drummond MF, Richardson WS, O’Brien BJ et al (1997) Users’ guides to the medical literature: XIII. How to use an article on economic analysis of clinical practice. Part A: are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 277:1552–1557
O’Brien BJ, Heyland D, Richardson WS et al (1997) Users’ guides to the medical literature: XIII. How to use an article on economic analysis of clinical practice. Part B: what are the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA 277:1802–1806
Martin DK, Pater JL, Singer PA (2001) Priority-setting decisions for new cancer drugs: a qualitative case study. Lancet 358:1676–1681
Acknowledgments
This project was supported by a fellowship grant (OF) from the Canadian Association of Medical Oncology/Canadian Institute of Health Research Fellowship.
Conflict of interest
All authors declare they have no relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freedman, O., Amir, E., Zimmermann, C. et al. Filling in the gaps: reporting of concurrent supportive care therapies in breast cancer chemotherapy trials. Support Care Cancer 19, 315–322 (2011). https://doi.org/10.1007/s00520-010-1069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-1069-5