Abstract
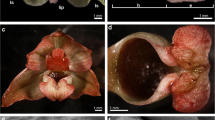
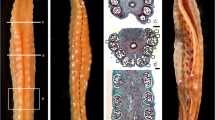
Pistil structure and composition are critical in recognizing and permitting the germination of suitable pollen grains. We have studied the structure of the different component tissues of the pistil, their organization and cytochemical features of olive flowers, Olea europaea L., at anthesis, an essential first step for understanding the processes of pollen-pistil interaction and fertilization. The pistil from olive cv. Picual trees is characterized by a wet bilobed stigma, a solid style and a bilocular ovary containing four ovules. The stigma is composed of external multicellular papillae and a non-papillate inner region of secretory cells. An exudate is observed on the surface of the papillae at anthesis, the moment when the flowers (first) open, but the anthers are not yet dehiscent. The inner secretory cells of the stigma and those of the stylar transmitting tissue are continuous, constituting a funnel-shaped zone which extends from within the stigma to the style base. The outer surface of the ovary and style epidermis is surrounded by a cuticle layer, while internally, the locule wall, formed by the innermost cells of the endocarp, consists of two layers of periclinally oriented cells with thicker cell walls. Starch granules are distributed differentially, concentrated most densely in the style (adjacent to the vascular bundles), in the distil region of the ovary, and in the micropylar ends of the ovules. Well-developed vascular bundles are present in the lower part of the stigma, the style and in the pericarp of the ovary. The histochemical identification of sugars and lipid substances within and around the vascular bundles suggests that they are involved in the transport of these materials. Ultrastructural observations confirm the presence of exudates on the papillar surface and confirm the secretory characteristics of the inner stigmatic cells. They also demonstrate marked differences in size, form, and vacuolar and cytoplasmic contents among the cells of the various style and upper ovary tissues. We provide the first detailed cytological description at anthesis of all the olive pistil tissues, indicating the structural and cytochemical basis for the pistil behavior which will transpire during the progamic phase.







Similar content being viewed by others
References
Alche JD, Rodriguez-Garcia MI (1988) Ultrastructural and cytochemical observations on intranuclear inclusions in Olea europaea. Inst Phys Conf Ser 93:65–66
Alche JD, Rodriguez-Garcia MI (1989) Application of X-ray microanalysis, diffraction and cytochemical techniques in the study of the structure and chemical composition of inclusions in Olea europaea leaves. Inst Phys Conf Ser 98:759–762
Alché JD, Fernandez MC, Rodriguez-Garcia MI (1994). Cytochemical features common to nucleoli and cytoplasmic nucleoloids of Olea europaea meiocytes: detection of rRNA by in situ hybridization. J Cell Sci 107:621–629
Aloni B, Karni L, Zaidman Z, Schaffer AA (1996) Changes of carbohydrates in pepper (Capsicum annuum L.) Flowers in relation to their abscission under different shading regimes. Ann Bot 78:163–168
Altamura Betti MM, Pasqua G, Mazzolani G (1982) Development of the female gametophyte in Olea europaea L. Ann Bot 40:111–117
Arbeloa A, Herrero M (1991) Development of the ovular structures in peach [Prunus persica (L.) Batsch]. New Phytol 118:527–534
Bartolini S, Guerriero R (1995) Self-compatibility in several clones of olive oil cv. ‘Leccino’. Adv Hortic Sci 9:71–74
Bigazzi M (1984) The occurrence of intranuclear inclusions in the Labiatae, Verbenaceae and Scrophulariaceae. Caryologia 37:269–292
Bronner R (1975) Simultaneous demonstration of lipids and starch in plant tissues. Stain Technol 50:1–4
Brun WA, Betts KJ (1984) Source: sink relations of abscissing and non abscissing soybean flowers. Plant Physiol 75:187–191
Ciampolini F, Cresti M, Kapil RN (1983). Fine structural and cytochemical characteristics of style and stigma in olive. Caryologia 36:211–230
Cresti M, Ciampolini F, Pacini E, Sarfatti G (1978) Phytoferritin in plastids of the style of Olea europaea L. Acta Bot Neerl 27:417–423
Cuevas J (2005) Autoincompatibilidad polen-pistilo, In: Rallo L, Barranco D, Caballero JM, Del Río C, Martín A, Tous J, Trujillo I (eds) Variedades de Olivo en España Junta de Andalucía, MAPA y Ediciones Mundi-Prensa Madrid, Spain, pp 301–308
Cuevas J, Polito VS (1997) Compatibility relationships in ‘Manzanillo’ olive. Hortic Sci 32:1056–1058
Cuevas J, Díaz-Hermoso AJ, Galián D, Hueso JJ, Pinillos V, Sola D, Polito VS (2001) Response to cross pollination and choice of pollinisers for the olive cultivars (Olea europaea L.) ‘Manzanilla de Sevilla’, ‘Hojiblanca’ and ‘Picual’. Olivae 85:26–32
De Graaf BHJ, Derksen JWM, Mariani C (2001) Pollen and pistil in the progamic phase. Sex Plant Reprod 14:41–55
De Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Springer, Berlin
Díaz A, Martín A, Rallo L, Barranco D, De la Rosa R (2006) Self incompatibility of ‘Arbequina’ and ‘Picual’ olives assessed by SSR markers. J Am Soc Hortic Sci 131:250–255
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:84–92
Feder NT, O’Brien P (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Felker FC, Robitaille HA, Hess FD (1983) Morphological and ultrastructural development and starch accumulation during chilling of sour cherry flower buds. Am J Bot 70:376–386
Fernández-Bolaños P, Frías L (1969) Autofertilidad y austerilidad en el olivo. Agricultura 443:150–151
Fernández MC, Rodriguez-Garcia MI (1988) Pollen wall development in Olea europaea L. New Phytol 108:91–99
Fernández MC, Rodriguez-Garcia MI (1989) Developmental changes in the aperture during pollen grain ontogeny in Olea europaea L. New Phytol 11:717–723
Fernández MC, Rodriguez-Garcia MI (1994) Pollen grain aperture in Olea europaea L. Rev Palaeobot Palynol 85:99–109
Fisher BB (1968) Protein staining of ribbonned epon sections for light microscopy. Histochemie 16:92–96
Fisher DB, Wu Y, Ku MSB (1992) Turnover of soluble proteins in the wheat sieve tube. Plant Physiol 100:1433–1441
Franceschi VR, Horner HT (1980) Calcium oxalate crystals in plants. Bot Rev 46:361–427
Fromm J, Hajirezaei M, Wilke I (1995) The biochemical response of electrical signalling in the reproductive system of Hibiscus plants. Plant Physiol 109:375–384
Ghosh S, Shivanna KR (1984) Structure and cytochemistry of the stigma and pollen–pistil interaction in Zephyranthes. Ann Bot 53:91–106
González MV, Coque M, Herrero M (1996) Pollen-pistil interaction in kiwifruit (Actinidia deliciosa; Actinidiaceae). Am J Bot 83:148–154
Herrero M (1992) Mechanisms in the pistil that regulate gametophyte population in peach (Prunus persica). In: Ottaviano E, Mulcahy DL, Sari Gorla M, Bergamini Mulcahy G (eds) Angiosperm pollen and ovule. Springer, New York, pp 377–381
Herrero M, Arbeloa A (1989) Influence of the pistil on pollen tube kinetics in peach (Prunus persica). Am J Bot 76:1441–1447
Herrero M, Dickinson HG (1979) Pollen-pistil incompatibility in Petunia hybrida: changes in the pistil following compatible and incompatible intraspecific crosses. J Cell Sci 36:1–18
Herrero M, Hormaza JI (1996) Pistil strategies controlling pollen tube growth. Sex Plant Reprod 9:343–347
Heslop-Harrison J, Heslop-Harrison Y (1985) Surfaces and secretions in the pollen-stigma interaction: a brief review. J Cell Sci Suppl 2:287–300
Heslop-Harrison Y, Shivanna KR (1977) The receptive surface of the angiosperm stigma. Ann Bot 41:1233–1258
Heslop-Harrison J, Heslop-Harrison Y, Reger BJ (1985) The pollen–stigma interaction in the grasses. Pollen tube guidance and the regulation of tube number in Zea mays L. Acta Bot Neerl 34:193–211
Jedrzejuk A, Szlachetka W (2005) Development of flower organs in common lilac (Syringa vulgaris L.) cv. Mme Florent Stepman. Acta Biol Cracov 47:41–52
Kadej AJ, Wilms HJ, Willemse MTM (1985) Stigma and stigmatoid tissue of Lycopersicon esculentum. Acta Bot Neerl 34:95–104
King JR (1938) Morphological development of the fruit of the olive. Hilgardia 11:437–458
Knox RB (1984) Pollen-pistil interactions. In: Linskens HF, Heslop-Harrison J (eds) Encyclopedia of plant physiology, new series, vol. 17. Springer, Berlin, pp 508–608
Knox RB, Williams EG, Dumas C (1986) Pollen, pistil, and reproductive function in crop plants. Plant Breeding Rev 4:9–79
Lavee S, Taryan J, Levin J, Haskal A (2002) The significance of cross-pollination for various olive cultivars under irrigated intensive growing conditions. Olivae 91:25–36
Madey E, Nowack LM, Thompson JE (2001) Isolation and characterization of lipid in phloem sap of canola. Planta 214:625–634
Majewska-Sawka A, Fernández MC, M´rani-Alaoui M, Münster A, Rodríguez-García MI (2002) Cell wall reformation by pollen tube protoplasts of olive (Olea europaea L.): comparison with pollen tube wall. Sex Plant Reprod 15:21–29
Marentes E, Grusak MA (1998) Mass determination of low-molecular-weight proteins in phloem sap using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Exp Bot 49:903–911
Martínez-Pallé E, Herrero M (1995) The ponticulus: a structure bridging pollen tube access to the ovule in Pistacia vera. Sex Plant Reprod 8:217–222
Martins PC, Cordeiro AM, Rapoport HF (2006) Flower quality in orchards of olive, Olea europaea L., cv. Morisca. Adv Hortic Sci 20:262–266
Moorkerjee S, Guerin J, Collins G, Ford C, Sedgley M (2005) Paternity analysis using microsatellite markers to identify pollen donors. Theor Appl Genet 111:1174–1182
Noher de Halac I, Fama G, Cismondi IA (1992) Changes in lipids and polysaccharides during pollen ontogeny in Oenothera anthers. Sex Plant Reprod 5:110–116
Pacini E, Juniper BE (1979a) The ultrastructure of pollen-grain development in olive (Olea europaea) 1. Proteins in the pore. New Phytol 83:157–164
Pacini E, Juniper BE (1979b) The ultrastructure of pollen-grain development in olive (Olea europaea) 2. Secretion by the tapetal cell. New Phytol 83:165–174
Rallo P, Rapoport HF (2001) Early growth and development of the olive fruit mesocarp. J Hortic Sci Biotech 76:408–412
Rapoport HF (2004) Botánica y morfología, In: D Barranco R Fernández-Escobar L Rallo (eds) El cultivo del olivo, 5th edn. Junta de Andalucia y Mundi-Prensa, Andalucia, pp 37–62
Reale L, Gromo S, Bonofiglio T, Orlandi F, Forniaceri M, Ferranti F, Romano B (2006) Reproductive biology of olive (Olea europaea L.) DOP Umbria cultivars. Sex Plant Reprod 19:151–161
Rodrigo J, Hormaza JI, Herrero M (2000) Ovary starch reserves and flower development in apricot (Prunus armeniaca). Physiol Plant 108:35–41
Rodríguez-García MI, Fernández MC (1990) Ultrastructural evidence of endoplasmic reticulum differentiation during the maturation of the olive pollen grain. Plant Syst Evol 171:221–231
Rodríguez-García MI, Alche JD, Fernández MC (1995) Immunocytochemical localization of allergenic protein (Ole e 1) in the endoplasmic reticulum of the developing olive pollen grain (Olea europaea L.). Planta 196:558–563
Rodríguez-García MI, M´rani-Alaoui M, Fernández MC (2003a) Behavior of storage lipids during pollen development and pollen grain germination of olive (Olea europaea L.). Protoplasma 221:237–244
Rodriguez-García MI, M´rani-Alaoui M, De la Flor Díaz J, Fernández MC (2003b) Observations on microtubules and nuclei motility in the pollen tube of olive (Olea europaea L.) pollen. Acta Biol Cracov 45:97–101
Sanzol J, Herrero M (2001) The effective pollination period in fruit trees. Sci Hortic 90:1–17
Sedgley M (1979) Structural changes in the pollinated and non-pollinated avocado stigma and style. J Cell Sci 38:49–60
Uwate WJ, Lin J (1981) Development of the stigmatic surface of Prunus avium L., sweet cherry. Am J Bot 68:1165–1176
Weintraub M, Ragetli HW, Schroeder B (1971). The protein composition of nuclear crystals in leaf cells. Am J Bot 58:182–190
Zinselmeier C, Westgate ME, Schussler JR, Jones RJ (1995) Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries. Plant Physiol 107:385–391
Acknowledgments
We thank Conchita Martínez-Sierra for excellent technical assistance. This work was supported by the Spanish Ministry of Education and Science (MEC), project AGL2003-00719 and a FPI fellowship to C.S. and a FPU fellowship to I.S. The “Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía” also provided financing for this study with project P06-AGR-01791.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott Russell.
I. Serrano and C. Suárez participated equally in this study and should both be considered as principal authors.
Rights and permissions
About this article
Cite this article
Serrano, I., Suárez, C., Olmedilla, A. et al. Structural organization and cytochemical features of the pistil in Olive (Olea europaea L.) cv. Picual at anthesis. Sex Plant Reprod 21, 99–111 (2008). https://doi.org/10.1007/s00497-008-0075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-008-0075-y