Abstract
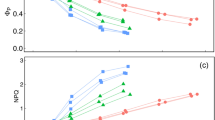
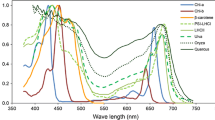
Pigments are known to modify the spectral properties of foliage, which in turn affect the amount of radiant energy stored by the plant canopy. Studies have shown that red pigments (anthocyanin) increase leaf absorptivity of solar radiation, but little is known about their effect on canopy net radiation and temperature. We hypothesized that increased absorptivity of solar radiation caused by red pigments would result in higher canopy temperature when compared to that of a green canopy. To better understand the role of red pigments on canopy net radiation and temperature, we conducted a study where we measured leaf spectral properties, canopy spectral reflectivity, stomatal conductance, net radiation, and leaf and canopy temperature of red and green cotton (Gossypium hirsutum L.) canopies. On average, albedo of the red canopy was 0.02 lower than that of the green canopy, and most of the differences in reflected solar irradiance were in near-infrared wavelengths. Red canopy had greater net radiation than the green canopy throughout the measurement period, and that was due to its lower albedo. Red canopy was about 1 °C warmer than the green canopy on average; however, computer simulation indicates that albedo was of secondary importance in controlling canopy temperature. Contrary to our hypothesis, results suggest that lower stomatal conductance in the red leaves was the main driver of canopy temperature differences between red and green canopies.










Similar content being viewed by others
References
Boldt JK, Meyer MH, Erwin JE (2014) Foliar anthocyanins: a horticultural review. Hortic Rev 42:209–251.
Campbell GS, Norman JM (1998) An introduction to environmental biophysics, 2nd edn. Springer, New York
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Chapra SC (2012) Applied numerical methods with Matlab® for engineers and scientists, 3rd edn. McGraw-Hill, New York
Close DC, Beadle CL (2003) The ecophysiology of foliar anthocyanin. Bot Rev 69(2):149–161
Field TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127:566–574
Gates DM, Keegan HJ, Schleter JC, Weidner VR (1965) Spectral properties of plants. Appl Opt 4(1):11–20
Gates DM (1980) Biophysical ecology. Springer, New York
Gausman HW (1982) Visible light reflectance, transmittance, and absorptance of differently pigmented cotton leaves. Remote Sens Environ 13(3):233–238
Grantz DA, Zhang XJ, Massman WJ, Delany A, Pederson JR (1997) Ozone deposition to a cotton (Gossypium hirsutum L.) field: stomatal and surface wetness effects during the California Ozone Deposition Experiment. Agric for Meteorol 85:19–31
Idso SB, Jackson RD, Ehrler WL, Mitchell ST (1969) A method for determination of infrared emittance of leaves. Ecology 50(5):899–902
Jones HG (2014) Plants and microclimate: A quantitative approach to environmental plant physiology, 3rd ed. Cambridge University Press, UK
Johnson, I.R. 2013. PlantMod: exploring the physiology of plant canopies. IMJ Software, Dorrigo, NSW, Australia. www.imj.com.au/software/plantmod.
Kanemasu ET, Tanner CB (1969) Stomatal diffusion resistance of snap beans. I. Influence of leaf-water potential. Plant Physiol 44:1547–1552.
Knipling EB (1970) Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens Environ 1(3):155–159
Monteith JL, Unsworth MH (2013) Principles of environmental physics, 4th. Academic Press, Elsevier, New York
Monteith JL (1959) The reflection of short-wave radiation by vegetation. Q J R Meteorol Soc 85:386–392
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59(14):3903–3911
Neill SO, Gould KS (2003) Anthocyanins in leaves: light attenuators or antioxidants? Funct Plant Biol 30:865–873
Radin JW, Lu Z, Pearcy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Pro Nat Acad Sci 91(15)7217–7221.
Riar R, Wells R, Edmisten K, Jordan D, Bacheler J (2013) Changes in cotton leaf pigmentation after abnormal exposure to sunlight. J Agric Res Dev 2(1):07–13
Rosenthal WD, Gerik TJ (1991) Radiation use efficiency among cotton cultivars. Agron J 83:655–658
Pietrini F, Ianelli MA, Massaci A (2002) Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant Cell Environ 25(10):1251–1259
Sestak Z, Catsky J, Jarvis PG (1971) Plant Photosynthetic Production, Manual of Methods. Dr. W. Junk, The Hague, Netherlands
Thornley JHM, Johnson IR (2000) Plant and crop modelling. Blackburn Press, New Jersey
Turner NC (1974) Stomatal behavior and water status of maize, sorghum, and tobacco under field conditions. II. At low soil water potential. Plant Physiol (53):360–365
Wells R (2001) Leaf pigment and canopy photosynthetic response to early flower removal in cotton. Crop Science 41:1522–1529
Zhang H, Hinze LL, Lan Y, Westbrook JK, Hoffmann WC (2012) Discriminating among cotton cultivars with varying leaf characteristics using hyperspectral radiometry. Trans ASABE 55(1):275–280
Acknowledgements
We would like to acknowledge Dr. C.W. Smith for providing the seeds of the materials used in this study and Dr. J.A. Landivar, Mrs. A. Maeda, and the personnel from the Texas AgriLife Research and Extension Center at Corpus Christi for maintaining the research plots during the study period.
Funding
Funding for this research was provided by Texas A&M AgriLife Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Highlights
• Red pigments increased leaf absorptivity of solar radiation.
• Red canopies had lower albedo and greater net radiation than green canopies.
• Red canopies were warmer than green canopies.
• Red leaves had lower stomatal conductance than green leaves.
• Stomatal conductance was the main mechanism controlling canopy temperature.
Rights and permissions
About this article
Cite this article
Carvalho, H.D.R., McInnes, K.J., Heilman, J.L. et al. Radiative balance and temperature of differently pigmented cotton canopies. Int J Biometeorol 66, 591–600 (2022). https://doi.org/10.1007/s00484-021-02221-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-021-02221-x