Abstract
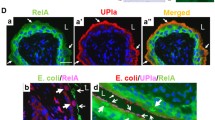
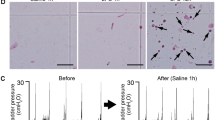
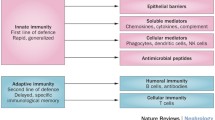
It has been shown previously that the intraction between uroepithelial cells (UEC) from healthy donors and adherent Escherichia coli suppresses bacterial growth in vitro. The following study was performed to investigate the nature of membrane signal transduction mechanisms involved in this process. UEC/E. coli cocultures were established in the presence of substances known to modulate transmembranous signals. Inhibition of calcium flux, either by calcium channel-blocking substances or by a calmodulin antagonist, depressed the antibacterial UEC function of “healthy” UEC. In contrast, receptor/ligand-induced stimulation of G-proteins, activation of the adenylate cyclase, and the increase in intracellular cyclic AMP levels by cytoplasmatic phosphodiesterase did not increase the antibacterial capacity of healthy UEC. However, the antibacterial function of defense-deficient UEC from patients with recurrent idiopathic urinary tract infection could be reconstituted by this treatment to almost normal levels. In conclusion, the antibacterial UEC defense function is activated by transmembranous signals from bacteria attached to the host cell surface. Activation involves the adenylate cyclase pathway. Activation of the phosphoinositol pathway may contribute to intracellular calcium fluxes.
Similar content being viewed by others
References
Bollgren I, Winberg J (1976) The periurethral aerobic flora in girls highly susceptible to urinary tract infections. Acta Paediatr Scand 65: 81–87
Cox CE, Hinman F (1961) Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J Urol 86: 739–743
Mannhardt W, Schofer O, Schulte-Wissermann H (1986) Pathogenic factors in recurrent urinary tract infections and renal scar formation in children. Eur J Pediatr 145: 330–336
Parsons CL (1986) Pathogenesis of urinary tract infections. Bacterial adherence, bladder defense mechanisms. Urol Clin North Am 13: 563–567
Schofer O, Ludwig K-H, Mannhardt W, Beetz R, Zepp F, Schulte-Wissermann H (1988) Antibacterial capacity of buccal epithelial cells from healthy donors and children with recurrent urinary tract infections. Eur J Pediatr 147: 229–232
Schulte-Wissermann H, Mannhardt W, Schwarz J, Zepp F, Bitter-Suermann D (1985) Comparison of the antibacterial effect of uroepithelial cells from healthy donors and children with asymptomatic bacteriuria. Eur J Pediatr 144: 230–233
Svanborg-Eden C, Jodal U (1979) Attachment of Escherichia coli to urinary sediment epithelial cells from urinary tract infection-prone and healthy children. Infect Immun 26: 837–840
Mannhardt W, Becker A, Putzer M, Bork M, Zepp F, Hacker J, Schulte-Wissermann H (1996) Host defense within the urinary tract. I. Bacterial adhesion initiates an uroepithelial defense mechanism. Pediatr Nephrol (in press)
Baddour LM, Christensen GD, Simpson WA, Beachey EH (1990) Microbial adherence. In: Mandell GL, Douglas RG, Bennett JE (eds) Principles and practice of infectious diseases. Churchill Livingstone, New York, pp 9–25
Linder H, Engberg I, Mattsby-Baltzer I, Janni K, Svanborg-Eden C (1988) Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect Immun 65: 1309–1313
Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ (1987) Regulation of transmembrane signaling by receptor phosphorylation. Cell 48: 913–922
Steadman R, Topley N, Jenner DE, Davies M, Williams JD (1988) Type 1 fimbriate Escherichia coli stimulates a unique pattern of degranulation by human polymorphonuclear leukocytes. Infect Immun 56: 815–822
Hadden JW (1988) Transmembrane signals in the activation of T lymphocytes by mitogenic antigens. Immunol Today 9: 235–239
Klausner RD (1987) Magnum PI: investigations of signal transduction. Cell 49: 439–442
Koyama S, Rennard SI, Leikauf GD, Shoji S, Essen S von, Claassen L, Robbins RA (1991) Endotoxin stimulates bronchial epithelial cells to release chemotactic factors for neutrophils. A potential mechanism for neutrophil recruitment, cytotoxicity, and inhibition of proliferation in bronchial inflammation. J Immunol 147: 4293–4301
Lucy JA, Bruckdorfer KR (1990) Membrane structure and function. In: Cohen RD, Lewis B, Alberti KGMM, Denman AM (eds) The metabolic and molecular basis of acquired disease. Baillere Tindall, London, pp 237–250
Masure HR, Shattuck RL, Storm DR (1987) Mechanisms of bacterial pathogenicity that involve production of calmodulin-sensitive adenylate cyclase. Microbiol Rev 51: 60–65
Peters JR (1990) Peptide hormone receptor function. In: Cohen RD, Lewis B, Alberti KGMM, Denman AM (eds) The metabolic and molecular basis of acquired disease. Baillere Tindall, London, pp 265–284
Stryer L (1990) Hormonwirkung. In: Stryer L (ed) Biochemie. Spektrum der Wissenschaft Verlagsgesellschaft, Heidelberg, pp 1011–1040
Zeuzem S, Schulz I, Caspary WF (1992) GTP-bindende Proteine in der Pathophysiologie innerer Erkrankungen. Dtsch Ärztebl 89: A1-2768–2774
Seamon KB, Daly JW (1986) Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Res 20: 1–151
Johnson JR (1991) Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4: 80–128
Leffler H, Svanborg-Eden C (1981) Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun 43: 920–929
Scheffer J, König W, Hacker J, Goebel W (1985) Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun 50: 271–278
Sissons JGP, Cohen J (1990) The response to infection. In: Cohen RD, Lewis B, Alberti KGMM, Denman AM (eds) The metabolic and molecular basis of acquired disease. Bailliere Tindall, London, pp 742–761
Svanborg C, Agace W, Hedges S, Lindstedt R, Svensson ML (1994) Bacterial adherence and mucosal cytokine production. Ann NY Acad Sci 730: 162–181
Damjanovich S, Szöllösi J, Tron L (1992) Transmembrane signalling in T-cells. Immunol Today 13: A12-A15
Ballou LR (1992) Sphingolipids and cell function. Immunol Today 13: 339–341
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mannhardt, W., Putzer, M., Zepp, F. et al. Host defense within the urinary tract. II. Signal trasducing events activate the urepithelial defense. Pediatr Nephrol 10, 573–577 (1996). https://doi.org/10.1007/s004670050163
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004670050163