Abstract
Background
Recurrent Clostridium difficile infection (rCDI) is a rising problem in children with chronic diseases. Fecal microbiota transplantation (FMT) is a recent alternative for rCDI patients who do not respond to conventional treatment. FMT could have an additional positive effect on the intestinal dysbiosis and accumulation of uremic retention molecules (URM) associated with chronic kidney disease (CKD). Our aim was to investigate the clinical efficacy of FMT for rCDI in children with CKD together with the effect on dysbiosis and URM levels.
Methods
We analyzed stool and blood samples before and until 3 months after FMT in 3 children between 4 and 8 years old with CKD and rCDI. The microbiome was analyzed by 16 s rRNA sequencing. URM were analyzed with ultra-performance liquid chromatography-tandem mass spectrometry. CRP and fecal calprotectin were analyzed as parameters for systemic and gut inflammation, respectively.
Results
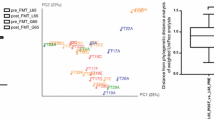
CDI resolved after FMT in all three without adverse events; one patient needed a second FMT. No significant effect on CRP and calprotectin was observed. Stool samples demonstrated a reduced richness and bacterial diversity which did not improve after FMT. We did observe a trend in the decrease of specific URM up to 3 months after FMT.
Conclusion
FMT is an effective treatment for rCDI in patients with CKD. Analysis of the microbiome showed an important intestinal dysbiosis that, besides a significant reduction in Clostridium difficile, did not significantly change after FMT. A trend for reduction was seen in some of the measured URM after FMT.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information





Similar content being viewed by others
Data availability
Data are available upon request to the corresponding author.
Abbreviations
- CD:
-
Clostridium difficile
- CDI:
-
Clostridium difficile Infection
- CKD:
-
Chronic kidney disease
- CNS:
-
Congenital nephrotic syndrome
- CRP:
-
C-reactive protein
- FMT:
-
Fecal microbiota transplantation
- GFR:
-
Glomerular filtration rate
- rCDI:
-
Recurrent clostridium difficile infection
- KRT:
-
Kidney replacement therapy
- SCFA:
-
Short-chain fatty acids
- SOT:
-
Solid organ transplantation
- URM:
-
Uremic retention molecules
References
Cozar-Llisto A, Ramos-Martinez A, Cobo J (2016) Clostridium difficile infection in special high-risk populations. Infect Dis Ther 5:253–269
Leffler DA, Lamont TJ (2015) Clostridium difficile infection. N Engl J Med 372:1539–1348
Dubberke ER, Burdette SD (2013) Clostridium difficile infections in solid organ transplantation. Am J Transplant 13(Suppl 4):42–49
Ciricillo J, Haslam D, Blum S, Kim MO, Liu C, Paulsen G, Courter J, Danziger-Isakov L (2016) Frequency and risks associated with Clostridium difficile-associated diarrhea after pediatric solid organ transplantation: a single-center retrospective review. Transpl Infect Dis 18:706–713
McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH (2018) Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66:987–994
O’Gorman MA, Michaels MG, Kaplan SL, Otley A, Kociolek LK, Hoffenberg EJ, Kim KS, Nachman S, Pfefferkorn MD, Sentongo T, Sullivan JE, Sears P (2018) Safety and pharmacokinetic study of fidaxomicin in children with clostridium difficile-associated diarrhea: a phase 2a multicenter clinical trial. J Pediatric Infect Dis Soc 7:210–218
Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, Sanguinetti M, Gasbarrini A (2015) Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 41:835–843
Hvas CL, Dahl Jørgensen SM, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, Erikstrup C, Dahlerup JF (2019) Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology 156:1324–1332
Shogbesan O, Poudel DR, Victor S, Jehangir A, Fadahunsi O, Shogbesan G, Donato A (2018) A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol 2018:1394379
Cheng YW, Phelps E, Ganapini V, Khan N, Ouyang F, Xu H, Khanna S, Tariq R, Friedman-Moraco RJ, Woodworth MH, Dhere T, Kraft CS, Kao D, Smith J, Le L, El-Nachef N, Kaur N, Kowsika S, Ehrlich A, Smith M, Safdar N, Misch EA, Allegretti JR, Flynn A, Kassam Z, Sharfuddin A, Vuppalanchi R, Fischer M (2019) Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am J Transplant 19:501–511
Friedman-Moraco RJ, Mehta AK, Lyon GM, Kraft CS (2014) Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant 14:477–480
Suchman K, Luo Y, Grinspan A (2022) Fecal microbiota transplant for Clostridioides Difficile infection is safe and efficacious in an immunocompromised cohort. Dig Dis Sci 67:4866–4873
Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A, Gordon S, Gluck M, Hohmann EL, Kao D, Kao JY, McQuillen DP, Mellow M, Rank KM, Rao K, Ray A, Schwartz MA, Singh N, Stollman N, Suskind DL, Vindigni SM, Youngster I, Brandt L (2014) Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109:1065–1071
Zhong S, Zeng J, Deng Z, Jiang L, Zhang B, Yang K, Wang W, Zhang T (2019) Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: a pediatric case report. Ital J Pediatr 45:116
Spinner JA, Bocchini CE, Luna RA, Thapa S, Balderas MA, Denfield SW, Dreyer WJ, Nagy-Szakal D, Ihekweazu FD, Versalovic J, Savidge T, Kellermayer R (2020) Fecal microbiota transplantation in a toddler after heart transplant was a safe and effective treatment for recurrent Clostridiodes difficile infection: a case report. Pediatr Transplant 24:e13598
Sabus A, Merrow M, Heiden A, Boster J, Koo J, Franklin ARK (2021) Fecal Microbiota transplantation for treatment of severe Clostridioides difficile Colitis in a pediatric patient with non-Hodgkin lymphoma. J Pediatr Hematol Oncol 43:e897–e899
Lynch SV, Pedersen O (2016) The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379
Evenepoel P, Poesen R, Meijers B (2017) The gut-kidney axis. Pediatr Nephrol 32:2005–2014
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83:308–315
Holle J, Bartolomaeus H, Löber U, Behrens F, Bartolomaeus TUP, Anandakumar H, Wimmer MI, Vu DL, Kuhring M, Brüning U, Maifeld A, Geisberger S, Kempa S, Schumacher F, Kleuser B, Bufler P, Querfeld U, Kitschke S, Engler D, Kuhrt LD, Drechsel O, Eckardt KU, Forslund SK, Thürmer A, McParland V, Kirwan JA, Wilck N, Müller D (2022) Inflammation in children with CKD linked to gut dysbiosis and metabolite imbalance. J Am Soc Nephrol 33:2259–2275
Cigarran Guldris S, Gonzalez Parra E, Cases Amenos A (2017) Gut microbiota in chronic kidney disease. Nefrologia 37:9–19
Mishima E, Fukuda S, Mukawa C, Yuri A, Kanemitsu Y, Matsumoto Y, Akiyama Y, Fukuda NN, Tsukamoto H, Asaji K, Shima H, Kikuchi K, Suzuki C, Suzuki T, Tomioka Y, Soga T, Ito S, Abe T (2017) Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int 92:634–645
Chung S, Barnes JL, Astroth KS (2019) Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. Adv Nutr 10:888–901
Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J (2016) Population-level analysis of gut microbiome variation. Science 352:560–564
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Vandeputte D, Kathagen G, D’hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, Wang J, Tito RY, De Commer L, Darzi Y, Vermeire S, Falony G, Raes J (2017) Quantitative microbiome profiling links gut community variation to microbial load. Nature 551:507–511
Prest EI, Hammes F, Kötzsch S, van Loosdrecht MC, Vrouwenvelder JS (2013) Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res 47:7131–7142
de Loor H, Poesen R, De Leger W, Dehaen W, Augustijns P, Evenepoel P, Meijers B (2016) A liquid chromatography-tandem mass spectrometry method to measure a selected panel of uremic retention solutes derived from endogenous and colonic microbial metabolism. Anal Chim Acta 936:149–156
Kolho KL, Korpela K, Jaakkola T, Pichai MV, Zoetendal EG, Salonen A, de Vos WM (2015) Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol 110:921–930
Vieira-Silva S, Sabino J, Valles-Colomer M, Falony G, Kathagen G, Caenepeel C, Cleynen I, van der Merwe S, Vermeire S, Raes J (2019) Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol 4:1826–1831
Kyle BD, Agbor TA, Sharif S, Chauhan U, Marshall J, Halder SLS, Ip S, Khan WI (2020) Fecal calprotectin, CRP and leucocytes in IBD patients: comparison of biomarkers with biopsy results. J Can Assoc Gastroenterol 4:84–90
Ihekweazu FD, Versalovic J (2018) Development of the pediatric gut microbiome: impact on health and disease. Am J Med Sci 356:413–423
Li F, Wang M, Wang J, Li R, Zhang Y (2019) Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol 9:206
Hu X, Ouyang S, Xie Y, Gong Z, Du J (2020) Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad Med 132:495–505
Yao Y, Cai X, Ye Y, Wang F, Chen F, Zheng C (2021) The role of microbiota in infant health: from early life to adulthood. Front Immunol 12:708472
Jiang S, Xie S, Lv D, Zhang Y, Deng J, Zeng L, Chen Y (2016) A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 109:1389–1396
Campos-Perez W, Martinez-Lopez E (2021) Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta Mol Cell Biol Lipids 1866:158900
Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ (2013) High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 4:125–135
Barba C, Soulage CO, Caggiano G, Glorieux G, Fouque D, Koppe L (2020) Effects of fecal microbiota transplantation on composition in mice with CKD. Toxins 12:741
Passmore IJ, Letertre MPM, Preston MD, Bianconi I, Harrison MA, Nasher F, Kaur H, Hong HA, Baines SD, Cutting SM, Swann JR, Wren BW, Dawson LF (2018) Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog 14:e1007191
Lau WL, Vaziri ND (2017) The leaky gut and altered microbiome in chronic kidney disease. J Ren Nutr 27:458–461
Gryp T, De Paepe K, Vanholder R, Kerckhof FM, Van Biesen W, Van de Wiele T, Verbeke F, Speeckaert M, Joossens M, Couttenye MM, Vaneechoutte M, Glorieux G (2020) Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int 97:1230–1242
Snauwaert E, Holvoet E, Van Biesen W, Raes A, Glorieux G, Vande Walle J, Roels S, Vanholder R, Askiti V, Azukaitis K, Bayazit A, Canpolat N, Fischbach M, Godefroid N, Krid S, Litwin M, Obrycki L, Paglialonga F, Ranchin B, Samaille C, Schaefer F, Schmitt CP, Spasojevic B, Stefanidis CJ, Van Dyck M, Van Hoeck K, Collard L, Eloot S, Shroff R (2019) Uremic toxin concentrations are related to residual kidney function in the pediatric hemodialysis population. Toxins 11:235
Snauwaert E, Van Biesen W, Raes A, Glorieux G, Van Bogaert V, Van Hoeck K, Coppens M, Roels S, Vande Walle J, Eloot S (2018) Concentrations of representative uraemic toxins in a healthy versus non-dialysis chronic kidney disease paediatric population. Nephrol Dial Transplant 33:978–986
Eloot S, Van Biesen W, Roels S, Delrue W, Schepers E, Dhondt A, Vanholder R, Glorieux G (2017) Spontaneous variability of pre-dialysis concentrations of uremic toxins over time in stable hemodialysis patients. PLoS One 12:e0186010
Xu L, Sinclair AJ, Faiza M, Li D, Han X, Yin H, Wang Y (2017) Furan fatty acids-beneficial or harmful to health? Prog Lipid Res 68:119–137
Greenhalgh K, Meyer KM, Aagaard KM, Wilmes P (2016) The human gut microbiome in health: establishment and resilience of microbiota over a lifetime. Environ Microbiol 18:2103–2116
Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini A, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66:569–580
Khanna S, Vazquez-Baeza Y, González A, Weiss S, Schmidt B, Muñiz-Pedrogo DA, Rainey JF, Kammer P, Nelson H, Sadowsky M, Khoruts A, Farrugia SL, Knight R, Pardi DS, Kashyap PC (2017) Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 5:55
Hourigan SK, Chen LA, Grigoryan Z, Laroche G, Weidner M, Sears CL, Oliva-Hemker M (2015) Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther 42:741–752
Acknowledgements
This work was co-funded by Vlaams Instituut voor Biotechnologie (VIB), the Rega Institute for Medical Research, Katholieke Universiteit (KU) Leuven, and the FWO/F.R.S.-FNRS under the Excellence of Science (EOS) program (MiQuant/30770923). The development of QMP analysis was funded by a KU Leuven CREA grant. J.V.C. was supported by a postdoctoral fellowship from the Research Foundation Flanders (FWO Vlaanderen-1236321N). We thank Prof. Ilse Hoffman for performing the FMT’s by colonoscopy. Thanks to the patients, parents and nurses of pediatric dialysis and transplantation who were involved in this study.
Author information
Authors and Affiliations
Contributions
J.V.C., C.C., N.K., and J.R. conceived the study objectives and study design. C.C. and N.K. coordinated recruitment and sample collection. L.R. carried out fecal microbial DNA extraction and sequencing. Fecal moisture, calprotectin, and cell counts were measured by C.C. Data preprocessing was done by J.V.C. Statistical analyses were designed and executed by J.V.C., A.S., J.R., C.C., and N.K. The draft manuscript was prepared by A.S., J.V.C., N.K., and J.R. and revised by all authors.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The approval was granted by the Ethics Committee of University Hospitals Leuven.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samaey, A., Vázquez-Castellanos, J.F., Caenepeel, C. et al. Effects of fecal microbiota transplantation for recurrent Clostridium difficile infection in children on kidney replacement therapy: a pilot study. Pediatr Nephrol 39, 1201–1212 (2024). https://doi.org/10.1007/s00467-023-06168-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06168-6