Abstract
Background
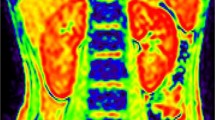
Vesicoureteral reflux (VUR) is a frequent cause of chronic kidney disease (CKD) in children. Using blood oxygenation level-dependent magnetic resonance imaging (BOLD-MRI), we measured cortical and medullary oxygenation in children with CKD due to VUR and compared the results to those obtained on healthy controls.
Method
The study population comprised 37 children (19 with CKD due to VUR and 18 healthy age-matched controls). BOLD-MRI was performed before and after furosemide treatment. MR images were analyzed with the region-of-interest (ROI) technique to assess the mean R2* values (=1/T2*) of the cortex and medulla of each kidney and with the concentric object (CO) technique that divides renal parenchyma in 12 equal layers.
Results
R2* values were significantly lower (corresponding to higher oxygenation) in the cortex and medulla of kidneys of children with CKD due to VUR than in those of the healthy controls (cortex 16.4 ± 1.4 vs. 17.2 ± 1.6 s−1 , respectively; medulla 28.4 ± 3.2 vs. 30.3 ± 1.9 s−1 , respectively; P < 0.05), and furosemide-induced changes in medullary R2* were smaller in the former than in the latter (−5.7 ± 3.0 vs. −6.9 ± 3.4 s−1, respectively; P < 0.05). Similar results were found with the CO technique. In children with a history of unilateral reflux (n = 9), the non-affected contralateral kidneys presented similar R2* values as the diseased kidneys, but their response to furosemide was significantly larger (−7.4 ± 3.2 vs. −5.7 ± 3.0, respectively; P = 0.05).
Conclusions
Chronic kidney disease due to VUR is not associated with kidney tissue hypoxia in children. The significantly larger furosemide-induced decrease in medullary R2* levels in the healthy group and unaffected contralateral kidneys of the VUR group points towards more intense renal sodium transport in these kidneys.


Similar content being viewed by others
References
Prasad PV, Edelman RR, Epstein FH (1996) Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94(12):3271–3275
Pruijm M, Hofmann L, Piskunowicz M, Muller ME, Zweiacker C, Bassi I, Vogt B, Stuber M, Burnier M (2014) Determinants of renal tissue oxygenation as measured with BOLD-MRI in chronic kidney disease and hypertension in humans. PLoS One 9:e95895
Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO (2003) Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46:1153–1160
Ries M, Basseau F, Tyndal B, Jones R, Deminière C, Catargi B, Combe C, Moonen CW, Grenier N (2003) Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen leveled pendent. J Magn Reson Imaging 17:104–113
Zhong Z, Arteel GE, Connor HD (1998) Cyclosporin a increases hypoxia and free radical production by rat kidneys: prevention by dietary glycine. Am J Physiol 1275:F595–F604
Bernhardt WM, Weisener MS, Weidemann A, Schmitt R, Weichert W, Lechler P, Campean V, Ong AC, Willam C, Gretz N, Eckardt KU (2007) Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol 170:830–842
Manotham K, Ongvilawan B, Urusopone P, Chetsurakran S, Tanamai J, Limkuansuwan P, Eiam-Ong S, Tungsanga K (2006) Intrarenal hypoxia in CKD patients, a BOLD MRI study. J Am Soc Nephrol 17:164A
Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC (2008) The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 19:780–788
Epstein FH, Prasad P (2000) Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int 57:2080–2083
International Reflux Study Committee (1981) Medical versus surgical treatment of primary vesicoureteric reflux. Pediatrics 67:392–400
Brakeman P (2008) Vesicoureteral reflux, reflux nephropathy, and end-stage renal disease. Adv Urol 508949
North American Pediatric Renal Trials andCollaborative Studies (2008) NAPRTCS 2008 annual report. Renal transplantation, dialysis, chronic renal insufficiency. North American Pediatric Renal Trials and Collaborative Studies, Boston
Chertin B, Solari V, Reen DJ, Farkas A, Puri P (2002) Up-regulation of angiotensin-converting enzyme (ACE) gene expression induces tubulointerstitial injury in reflux nephropathy. Pediatr Surg Int 18(7):635–639
Goonasekera CDA, Dilon MJ (1999) Hypertension in reflux nephropathy. BIJ Int 83:1–12
Brenner BM, Meyer TW, Hostetter TH (1982) Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307:652–659
Dunn BR, Anderson S, Brenner BM (1986) The hemodynamic basis of progressive renal disease. Semin Nephrol 6:122–138
Remuzzi G, Bertani T (1998) Pathophysiology of progressive nephropathies. N Engl J Med 339:1448–1456
Heyman SN, Khamaisi M, Rosen S, Rosenberger C (2008) Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol 28(6):998–1006
Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, Moxey-Mims MM, Warady BA, Furth SL, Wong CS (2015) Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10(4):571–577
Schainuck LI, Striker GE, Cutler RE, Benditt EP (1970) Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1:631–641
Eddy AA (2005) Progression in chronic kidney disease. Adv Chronic Kidney Dis 12:353–365
Fine LG, Orphanides C, Norman JT (1998) Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl 65:S74–S78
Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Möbius TE (1985) International system of radiographic grading of vesicoureteric reflux: international reflux study in children. Pediatr Radiol 15:105–109
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI (2014) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63:713–735
O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P, European Society of Hypertension Working Group on Blood Pressure Monitoring (2003) European society of hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 21:821–848
Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M, Bochud M (2015) Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension 66:85–92
Gao A, Cachat F, Faouzi M, Bardy D, Mosig D, Meyrat BJ, Girardin E, Chehade H (2013) Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int 83(3):524–530
Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, Burnier M, Vogt B (2010) Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 55:1116–1122
Pruijm M, Hofmann L, Vogt B, Muller ME, Piskunowicz M, Stuber M, Burnier M (2013) Renal tissue oxygenation in essential hypertension and chronic kidney disease. Int J Hypertens 2013:696598. doi:10.1155/2013/696598
Piskunowicz M, Hofmann L, Zuercher E, Bassi I, Milani B, Stuber M, Narkiewicz K, Vogt B, Burnier M, Pruijm M (2015) A new technique with high reproducibility to estimate renal oxygenation using BOLD-MRI in chronic kidney disease. Magn Reson Imaging 33:253–261
Vakilzadeh N, Muller ME, Forni V, Milani B, Hoffman L, Piskunowicz M, Maillard M, Zweiacker C, Pruijm M, Burnier M (2015) Comparative effect of a renin inhibitor and a thiazide diuretic on renal tissue oxygenation in hypertensive patients. Kidney Blood Press Res 40(5):542–554
Basile DP, Donohoe D, Roethe K, Osborn JL (2001) Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Ren Physiol 281:F887–F899
Epstein FH, Veves A, Prasad PV (2002) Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care 25:575–578
Rosenberger C, Khamaisi M, Abassi Z (2008) Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73:34–42
Welch WJ (2006) Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol 33:1002–1005
Kairaitis LK, Wang Y, Gassman M, Tay Y-C, Hamlyn Harris DC (2005) HIF-1a expression follows microvascular loss in advanced murine adriamycin nephrosis. Am J Physiol Ren Physiol 288:F198–F206
Xin-Long P, Jing-Xia X, Jian-Yu L, Song W, Xin-Kui T (2012) A preliminary study of blood-oxygen-level-dependent MRI in patients with chronic kidney disease. Magn Reson Imaging 30(3):330–335
Djamali A, Sadowski EA, Muehrer RJ, Reese S, Smavatkul C, Vidyasagar A, Fain SB, Lipscomb RC, Hullett DH, Samaniego-Picota M, Grist TM, Becker BN (2007) BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Ren Physiol 292(2):F513–F522
Manotham K, Ongvilawan B, Urusopone P, Chetsurakarn S, Tanamai J, Limkuansuwan P, Tungsanga K, Eiam-Ong S (2012) Angiotensin II receptor blocker partially ameliorated intrarenal hypoxia in chronic kidney disease patients:a pre-/post-study. Intern Med J 42(4):e33–e37
Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H (2011) Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22(8):1429–1434
Prasad PV, Thacker J, Li LP, Haque M, Li W, Koenigs H, Zhou Y, Sprague SM (2015) Multi-parametric evaluation of chronic kidney disease by MRI: a preliminary cross-sectional study. PLoS One 10(10):e0139661
Siddiqi L, Hoogduin H, Visser F, Leiner T, Mali WP, Blankestijn PJ (2014) Inhibition of the renin-angiotensin system affects kidney tissue oxygenation evaluated by magnetic resonance imaging in patients with chronic kidney disease. J Clin Hypertens 16(3):214–218
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81(7):684–689
Neugarten J, Golestaneh L (2014) Blood oxygenation level-dependent MRI for assessment of renal oxygenation. Int J Nephrol Renov Dis 7:421–435
Malvezzi P, Bricault I, Terrier N, Bayle F (2009) Evaluation of intrarenal oxygenation by blood oxygen level-dependent magnetic resonance imaging in living kidney donors and their recipients: preliminary results. Transplant Proc 41(2):641–644
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research project was approved by the local institutional review committee (Ethical Committee of Canton de Vaud, Switzerland) and conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each participant and parents.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding source
This study was supported by a grant from the Swiss National Science Foundation (FN 32003B-149309).
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Rights and permissions
About this article
Cite this article
Chehade, H., Milani, B., Ansaloni, A. et al. Renal tissue oxygenation in children with chronic kidney disease due to vesicoureteral reflux. Pediatr Nephrol 31, 2103–2111 (2016). https://doi.org/10.1007/s00467-016-3419-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3419-0