Abstract
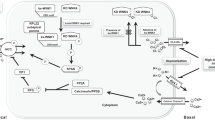
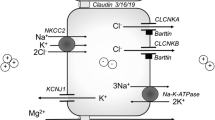
Gordon Syndrome (GS) is a rare familial hypertension syndrome with a characteristic hyperkalaemia which distinguishes it from other syndromic forms of hypertension that typically cause hypokalaemia. Patients with GS respond to aggressive salt-restriction or relatively small doses of thiazide diuretics, which suggests that activation of the thiazide-sensitive Na/Cl cotransporter (NCC) in the distal nephron is to blame. However, the mechanism has proved to be complex. In 2001, mutations in genes encoding two serine/threonine kinases, WNK1 and WNK4, were identified as causing GS. However, it took several years to appreciate that these kinases operated in a cascade with downstream serine/threonine kinases (SPAK and OSR1) actually phosphorylating and activating NCC and the closely related cotransporters NKCC1 and NKCC2. The hyperkalaemia in GS arises from an independent action of WNK1/WNK4 to reduce cell-surface expression of ROMK, the secretory K-channel in the collecting ducts. However, mutations in WNK1/4 are present in a small minority of GS families, and further genes have emerged (CUL3 and KLHL3) that code for Cullin-3 (a scaffold protein in an ubiquitin–E3 ligase) and an adaptor protein, Kelch3, respectively. These new players regulate the ubiquitination and proteasomal degradation of WNK kinases, thereby adding to the complex picture we now have of NCC regulation in the distal nephron.



Similar content being viewed by others
References
Paver W, Pauline G (1964) Hypertension and hyperpotassaemia without renal disease in a young male. Med J Aust 2:305–306
Gordon RD, Geddes RA, Pawsey CG, O’Halloran MW (1970) Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas Ann Med 19:287–294
Toka HR, Koshy JM, Hariri A (2013) The molecular basis of blood pressure variation. Pediatr Nephrol 28:387–399
Achard JM, Disse-Nicodeme S, Fiquet-Kempf B, Jeunemaitre X (2001) Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (Gordon syndrome). Clin Exp Pharmacol Physiol 28:1048–1052
Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z (2002) Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87:3248–3254
Gordon RD, Hodsman GP (1986) The syndrome of hypertension and hyperkalaemia without renal failure: long term correction by thiazide diuretic. Scott Med J 31:43–44
Mansfield TA, Simon DB, Farfel Z, Bia M, Tucci JR, Lebel M, Gutkin M, Vialettes B, Christofilis MA, Kauppinen Makelin R, Mayan H, Risch N, Lifton RP (1997) Multilocus linkage of familial hyperkalaemia and hypertension, pseudohypoaldosteronism type II, to chromosomes 1q31–42 and 17p11–q21. Nat Genet 16:202–205
O’Shaughnessy KM, Fu B, Johnson A, Gordon RD (1998) Linkage of Gordon’s syndrome to the long arm of chromosome 17 in a region recently linked to familial essential hypertension. J Hum Hypertens 12:675–678
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112
Verissimo F, Jordan P (2001) WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20:5562–5569
Uchida S, Sohara E, Rai T, Sasaki S (2014) Regulation of with-no-lysine kinase signaling by Kelch-like proteins. Biol Cell 106:45–56
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T (2013) The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 45:111–122
Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP (2004) WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc Natl Acad Sci USA 101:2064–2069
Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP (2003) WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl–transporting epithelia. Proc Natl Acad Sci USA 100:663–668
Yang CL, Angell J, Mitchell R, Ellison DH (2003) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111:1039–1045
Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP (2003) Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100:680–684
Golbang AP, Murthy M, Hamad A, Liu CH, Cope G, Van’t Hoff W, Cuthbert A, O’Shaughnessy KM (2005) A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension 46:295–300
Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H (2010) WNK4 enhances the degradation of NCC through a Sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21:82–92
Vitari AC, Deak M, Morrice N, Alessi DR (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391:17–24
Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT (2014) The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal 7:re3
Rosenbaek LL, Kortenoeven MLA, Aroankins TS, Fenton RA (2014) Phosphorylation decreases ubiquitylation of the Thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289:13347–13361
Glover M, Mercier Zuber A, Figg N, O’Shaughnessy KM (2010) The activity of the thiazide-sensitive Na(+)-Cl(-) cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88:986–995
Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G (2006) The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281:28755–28763
Yang S-S, Fang Y-W, Tseng M-H, Chu P-Y, Yu I-S, Wu H-C, Lin S-W, Chau T, Uchida S, Sasaki S, Lin Y-F, Sytwu H-K, Lin S-H (2013) Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24:1587–1597
Chavez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castaneda-Bueno M, Vazquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J (2014) WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension. doi:10.1161/HYPERTENSIONAHA.114.04036
Cope G, Golbang A, Murthy M, Hamad A, Liu CH, Hoff WV, Cuthbert A, O’Shaughnessy KM (2006) WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17:1867–1874
Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP (2003) WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35:372–376
Lazrak A, Liu Z, Huang CL (2006) Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103:1615–1620
Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J (2013) WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci USA 110:14366–14371
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X (2012) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44:456–460
Glover M, Ware JS, Henry A, Wolley M, Walsh R, Wain LV, Xu S, Van’t Hoff WG, Tobin MD, Hall IP, Cook S, Gordon RD, Stowasser M, O’Shaughnessy KM (2014) Detection of mutations in KLHL3 and CUL3 in families with FHHt (familial hyperkalaemic hypertension or Gordon’s syndrome). Clin Sci (Lond) 126:721–726
Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S (2014) Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. doi:10.1093/hmg/ddu217
Disse-Nicodeme S, Desitter I, Fiquet-Kempf B, Houot AM, Stern N, Delahousse M, Potier J, Ader JL, Jeunemaitre X (2001) Genetic heterogeneity of familial hyperkalaemic hypertension. J Hypertens 19:1957–1964
Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S (1999) Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880–883
Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD (2012) Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell Metab 16:462–472
Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, Shi X, Pan L, Rampersaud E, Shen H, Kim JD, Subramanya AR, Steinle NI, Parsa A, Ober CC, Welling PA, Chakravarti A, Weder AB, Cooper RS, Mitchell BD, Shuldiner AR, Chang YP (2009) Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA 106:226–231
Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, Kahle KT (2014) The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal 7(334):re3
Siew K, O’Shaughnessy KM (2013) Extrarenal roles of the with-no-lysine[K] kinases (WNKs). Clin Exp Pharmacol Physiol 40:885–894
Mori T, Kikuchi E, Watanabe Y, Fujii S, Ishigami-Yuasa M, Kagechika H, Sohara E, Rai T, Sasaki S, Uchida S (2013) Chemical library screening for WNK signalling inhibitors using fluorescence correlation spectroscopy. Biochem J 455:339–345
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Shaughnessy, K.M. Gordon Syndrome: a continuing story. Pediatr Nephrol 30, 1903–1908 (2015). https://doi.org/10.1007/s00467-014-2956-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2956-7