Abstract
Receptor activator of NF-kB ligand (RANKL) and osteoprotegerin (OPG) play key roles in the pathogenesis of glucocorticoid-induced osteoporosis (GIO). The aim of our study was to determine whether the cumulative glucocorticoid dose (CGCS) in children with idiopathic nephrotic syndrome (INS) has any effect on the concentration of serum RANKL and OPG and the RANKL/OPG ratio. The study population consisted of 90 children with INS, aged 3–20 years, who were treated with GCS. These children were divided into two groups according to the CGCS: low (L) <1 g/kg body weight (BW) and high (H) ≥1 g/kg BW, respectively. The control group (C) consisted of 70 healthy children. RANKL concentration was observed to be significantly higher and OPG significantly lower in INS children than in the reference group: 0.21 (range 0.01–1.36) versus 0.15 (0–1.42) pmol/l (p < 0.05), respectively, and 3.76 (1.01–7.25) versus 3.92 (2.39–10.23) pmol/l (p < 0.05), respectively. The RANKL/OPG ratio was significantly higher in INS children (p < 0.01). The concentration of RANKL, similar to the RANKL/OPG ratio, was significantly higher in Group H children than in Group L children: 0.46 (0.02–1.36 ) versus 0.19 (0.01–1.25) (p < 0.01) and 0.14 (0.01–0.71) versus 0.05 (0.002–0.37) (p < 0.01), respectively. The concentration of OPG was similar in both groups. There was a positive correlation between CGCS and the concentration of sRANKL as well as the RANKL/OPG ratio (in both cases r = 0.33, p < 0.05). Based on these results, we suggest that long-term exposure to GCS results in a dose-dependent increase in serum RANKL concentration and the RANKL/OPG ratio, but not in the level of serum OPG.
Similar content being viewed by others
Introduction
Glucocorticoid-induced osteoporosis (GIOP) was described for the first time in the early part of the last century by Cushing, who noticed that patients with endogenous hypercortisolism were relatively more susceptible than healthy individuals to bone fracture [1]. However, the relation between exposure to exogenous glucocorticoids (GCS) and skeletal disturbances was the subject of heated discussion for many years. At the present time, GIOP is considered to be one of the most serious complications of the pharmacological use of GCS.
Studies in adults have shown that GCS cause rapid and dose-dependent bone loss [2]. During childhood and adolescence, skeletal changes result in sex- and maturation-specific increases in bone dimensions and density. Children therefore seem to be predisposed to the effects of GCS on bone formation, leading to possible compromises in peak bone mass. Decreased bone mineral density (BMD) has already been described in many of the different pediatric disorders requiring therapy with GCS, including juvenile rheumatoid arthritis, inflammatory bowel disease, and systemic lupus erythematosus [3]. However, the data that have been published on BMD in children with nephrotic syndrome (NS) who have been treated with GCS are equivocal. Some researchers consider that, in contrast to other medical conditions requiring long-term GCS therapy, BMD in steroid-sensitive idiopathic nephrotic syndrome (INS) is stable because the symptoms remit soon after the initiation of GCS treatment and the time of exposure to GCS is relatively short [4].
Dual-energy X-ray absorptiometry (DXA) is the most widely applied method for evaluating bone mineral content (BMC) and BMD in patients of all ages, but its use in pediatric patients poses a problem in terms of technical aspects. A second issue is that height-, gender-, and age-adjusted normative data are available for the proper interpretation of results in children with normal glomerular filtration rate (GFR), but these should not be applied to children with chronic kidney disease who standardly show growth retardation and hormonal disturbances. Consequently, new, easy-to-use markers are necessary.
In 1997, Simonet et al. [5] reported a novel set of proteins in the tumor necrosis factor (TNF)/TNF receptor families that were required for the control of bone remodeling. These receptors, namely, receptor activator for nuclear factor kappa B (RANK), osteoprotegerin (OPG), and the RANK ligand (RANKL), were identified as forming a critical molecular triad that controled bone remodeling. Subsequent study confirmed that the balance between the two peptides produced by osteoblasts, OPG and RANKL, is critical for the bone resorption process [6]. The binding of RANKL to its receptor (RANK) induces differentiation, activation, and the prevention of osteoclast apoptosis, leading to enhanced bone resorption and bone loss [7]. The effects of RANKL can be neutralized by its decoy receptor OPG, which is an inhibitor of osteoclastic bone resorption [8]. GCS disturb the function of osteoblasts and, as a result, they suppress bone formation by decreasing the number of cells of osteoblastic lineage and inhibiting their differentiation [9]. In contrast, GCS stimulate the differentiation and function of osteoclasts, which are cells derived from the monocyte/macrophage family, differentiating under the control of two cytokines: macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) [10]. GCS increase the expression of M-CSF and RANKL and decrease the expression of RANKL soluble decoy receptor-OPG produced by the osteoblasts [11]. Consequently, OPG blocks the binding between RANKL and RANK, thereby playing the role of the major inhibitor of osteoporosis. The RANKL/OPG ratio seems to be the key mechanism in the pathogenesis of GIOP [10].
Few clinical reports have been published on the role of serum RANKL and OPG levels in the diagnosis of glucocorticoid-induced osteoporosis. Oelzner et al. [12] demonstrated that high serum levels of RANKL were associated with osteoporosis in patients with rheumatoid arthritis, but that influences of age and gender must also be considered. Turk et al. [13] found that free sRANKL and OPG showed a highly inverse relationship in patients with reduced bone density in the course of Crohn disease but not in those with healthy skeleton. There have been no published reports on the role of the RANKL–OPG system in the diagnosis of GC-induced reduction in BMD in nephrotic children.
The study reported here is a retrospective study in which we investigated whether cumulative glucocorticoid dose (CGCS) in children with INS has any influence on the concentration of RANKL and OPG and on the RANKL/OPG ratio.
Materials and methods
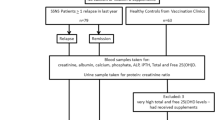
The study cohort consisted of 90 children and adolescents (males 51, females 39), aged 3–20 years, who had been diagnosed with INS, according to the definition of the International Society of Kidney Disease in Children [14], and who had been treated by or had consulted the Department of Pediatrics and Nephrology and followed up for 7 years. Inclusion criteria were age 3–20 years, normal GFR (>90 ml/min/1.73 m2), absence of clinical and laboratory findings of a systemic disease, ≥6-months interval since NS diagnosis, a history of systemic GCS therapy for NS, and remission of NS at the time of examination.
The children were divided to two groups according to CGCS, namely, a low [Group L; <1 g/kg body weight (BW)] and high (Group H; ≥1 g/kg BW) CGCS group and according to GCS treatment at the time of the study: GCS (+) and GCS (−).
The control group consisted of 70 healthy, age- and sex-matched children and adolescents diagnosed in our Department to have miction disturbances, who were not on medications [15]. Participants were ineligible if they had a height or body mass index (BMI) below the third percentile for age and sex or a history of illnesses or medications that might affect growth, nutritional status, pubertal development, or bone accrual. The distributions of age, sex, height, and BMI did not differ across the reference groups contributing data for each measurement.
All of the children with INS were treated with the standard initial therapy, consisting of daily prednisone 60 mg/m2 body surface area for 4 weeks, followed by 40 mg/m2 on alternate days for 2 weeks, and then with various tapering regimens on alternate days. Relapses were treated with daily prednisone 60 mg/m2 until remission was achieved, and then with the standard initial therapy just described. Based on a survey of the medical historys, more than 60% of the children had required alternative therapy: cyclosporine A (CSA) in 33/90 (36%) of patients, cyclophosphamide in 35/90 (39%) patients, chlorambucil in 5/90 (6%) patients, and azathioprin in 2/90 (2%) patients. At the moment of the examination, 49 (54%) patients were not receiving any drug, and 41 (46%) were receiving prednisone at a dose ranging from 0.1 to 2 mg/kg BW/24 h.
The medical charts were reviewed for date of diagnosis of NS, prior therapies, and current medications. Patients and their parents were interviewed at the study visit to confirm current medications and the date of the last dose of GCS therapy.
The protocol was approved by the Bioethics Committee of the Medical University of Białystok in accordance with the Declaration of Helsinki.
Urine and blood samples were drawn from all patients and children in the control group to measure serum RANKL, OPG, creatinine, albumin, and cholesterol concentrations. All samples were taken after 12-h of night fasting. Urinalysis was performed to assess protein and creatinine level and to determine the protein/creatinine ratio in the morning sample. The glomerular filtration rate (ml/min/1.73 m2) was estimated by the Schwartz formula.
A careful clinical history and physical examination were performed in all children. Height, weight, and BMI were expressed as centile for age, with reference to published standards [16]. The stage of pubertal development was determined using a validated self-assessment questionnaire and classified according to the method of Tanner.
Serum samples were frozen at −80°C and determined after sufficient samples had been collected for testing using one kit, usually after 12–18 months.
Both OPG and RANKL serum concentrations were determined using an enzyme-linked immunosorbent assay (ELISA) method according to the instructions given by the manufacturer (Biomedica Medizinprodukte GMBH, Vienna, Austria). The detection limit of OPG and RANKL was 0.14 and 0.02 pmol/l, respectively. The intra- and inter-assay coefficients of variation (CV) for both markers were: <10 and <10%, respectively.
Bone mineral content (g) and BMD (g/cm2) in the total body and lumbar spine were measured by DXA (DPX-L, ver. 1.3z GE-Lunar Radiation Corp., Madison, WI). The CV was 1.1% based on repeated scans in 32 pediatric subjects. The normative data published by Płudowski et al. [17] in healthy Polish children were used to analyze the bone DXA results.
Statistical methods
The data were analyzed using the Statistica ver. 8.0 package (StatSoft, Tulsa, OK). The adequacy of the parameters to normal distribution was tested using the Shapiro–Wilk test. Student’s t test was used to compare independent data of normal distribution, applying the Mann–Whitney U test when the variables did not follow a normal distribution. The data with skewed distribution were log-transformed before starting the statistical tests. Correlations between clinical and laboratory parameters were tested by Spearman correlation coefficients. Logistic regression was used to determine odds ratios (OR) with 95% confidence intervals (CI) for the effects of each predictor variable on the outcomes (increased serum RANKL level and RANKL/OPG ratio or decreased OPG level). The multivariate logistic regression model yielded an OR and 95% CI for each factor in the model. All p values are two sided, with p < 0.05 considered to be statistically significant.
Results
The anthropometric and biochemical characteristics of the study subjects with INS and the reference group are summarized in Table 1. The age in both groups was similar, but the proportion of males was significantly greater among the INS children, which is consistent with the demographics of childhood INS. Pubertal maturation, assessed by Tanner stage, was not significantly delayed in the NS children compared with controls. We found significantly lower height Z-scores, greater BMI Z-scores, and a greater prevalence of obesity and short stature in the INS group, as a result of chronic GCS treatment. All INS children were in remission of NS syndrome. The serum creatinine and albumin levels did not differ between the INS children and the controls.
In INS children, serum level of RANKL was increased and the level of OPG was decreased in comparison to the reference group (p < 0.05). The RANKL/OPG ratio was also statistically significant different between the two groups (p < 0.01); however, as the levels of RANKL and OPG differed between the groups, the statistically significant difference in their ratio is not independent.
Table 2 shows the disease characteristics of patients with INS according to CGCS (Group H and L, respectively). The age at diagnosis of NS in the children of both groups was comparable (p > 0.05), but the interval since diagnosis was significantly longer in Group H children (p < 0.01). Similarly, the number of relapses was almost threefold higher in Group H children. At the time of the study visit, 34% of Group L children and 60% of Group H children were taking oral GCS therapy. The cumulative dose of prednisone (in g/kg BW) was fivefold higher in Group H children than in Group L children (p < 0.01).
We then analyzed the differences in sRANKL concentrations and in the RANKL/OPG ratio between Group H and L children. The concentration of RANKL was significantly higher in the group of children treated with a high dose of CGCS (Group H) than in the group of children with a low exposure to GCS (Group L): 0.46 (range: 0.02–1.36) versus 0.19 (0.01–1.25) pmol/l (p < 0.01). The concentrations of OPG (Group H: median 3.72, range 1.00–7.24; Group L: 3.77, 2.19–6.34) were similar in both groups. The RANKL/OPG ratio was almost threefold higher in Group H children than in Group L children: 0.14 (0.01–0.71) versus 0.05 (0.002–0.37), respectively (p < 0.01).
The serum levels of RANKL and OPG and the RANKL/OPG ratio were compared between children who were being treated with GCS at the time of their examination [GCS (+) group] and those who had not received any treatment [GCS (−) group] (Fig. 1). Children being treated with GCS had a statistically significant higher serum concentration of RANKL (median 0.37 pmol/l, range 0.05–1.35 pmol/l) than those in the GCS (−) group (0.18 pmol/l, 0.01–0.85 pmol/l) (p < 0.05). Similar results were shown for the RANKL/OPG ratio. The serum level of OPG in the GCS (+) group was significantly lower (3.56 pmol/l, 1.01–7.24 pmol/l) than that in the GCS (−) group (3.99 pmol/l, 2.19–6.34 pmol/l) (p < 0.01).
Logistic regression analysis was performed to evaluate the risk factors. The incidence of increased serum RANKL and RANKL/OPG ratio and decreased serum OPG was not significantly associated with the variables sex (female), age >10 years, Tanner stage (5th), height <3 centile, overweight >90 centile, and age at onset >8 years.
Multivariate logistic regression analysis (data not shown) of those clinical factors that might predict an increase in the serum RANKL/OPG ratio in nephrotic patients identified number of relapses >10 (OR 2.76, 95% CI 1.07–7.13; p < 0.05) and cumulative steroid dose >1 g/kg BW (OR 2.28, 95% CI 0.89–5.87 (p < 0.05). The associations with serum RANKL did not achieve statistical significance (p > 0.05).
We also showed that the serum RANKL concentration and RANKL/OPG ratio in the INS children were significantly and positively correlated with GCS (in both cases r = 0.33, p < 0.05). Each gram of CGCS used in the therapy resulted in an increase in the RANKL serum concentration about 0.13 pmol/l and in the RANKL/OPG ratio of 0.05 (Fig. 2).
Using linear regression, we assessed whether the decrease in height and increased BMI had an influence on the association between the CGCS and serum RANKL and RANKL/OPG ratio. This analysis revealed that the addition of height <3 centile or BMI >90 centile increased the association between these parameters.
The effect of an alternative treatment in NS children was also analyzed. The children included to this study were not receiving any other immunosuppressant drugs at the moment of examination. However, 33 of the 90 NS children included in our study had been treated with CSA in the past, which is why we assessed the risk of increased serum RANKL concentrations (above the median value of the NS group) and decreased serum OPG concentrations (below the median value of the NS group). We found that the OR of an increased concentration of RANKL in patients treated with CSA was 1.83 (95% CI 0.77–4.34), and the relative risk of an increased serum RANKL concentration in NS children treated with CSA was 1.33 (0.8–1.9). Consequently, treatment with CSA did not influence the serum OPG level.
The most important focus of our study was to analyze the association between the examined parameters and the BMD in NS children. Unfortunately, in our analysis, we were able to assess the BMD in only 24/40 children treated with high doses of GCS. The median BMD Z-score in this group was −0.79 (range −3.87–0.49). We confirmed GIOP in only two of 24 children, but there was a low normal Z-score BMD in 45.8%. The OR of an increased concentration of RANKL in patients with a low normal Z-score was 6 (95% CI 1.08–33.3).
We found a statistically significant correlation between the BMD Z-score and serum RANKL (r = −0.44, p < 0.05). There was no correlation between the BMD Z-score and serum OPG level (Fig. 3).
Discussion
To the best of our knowledge, our study is the first to demonstrate the influence of the total cumulative dose of GCS on serum RANKL and OPG concentrations and also on the RANKL/OPG ratio in children and adolescents treated with GCS due to steroid-sensitive or steroid dependent NS and to compare the data to those obtained from concurrent reference subjects (controls).
Our results show that in our study cohort, the circulating OPG level was reduced and circulating RANKL level was increased in INS patients compared to those of a reference group. The most significant difference was found for the RANKL/OPG ratio. The RANKL-to-OPG balance is of critical importance for bone remodeling and the preservation of bone mass. We confirmed that osteoclast activity is likely to depend on the relative balance of RANKL and OPG, which is why it has been suggested that the RANKL-to-OPG ratio in serum—rather than individual protein concentrations—is the critical factor for determining osteoclastic activation at the bone level [6]. Alternation in this ratio has been confirmed in several bone loss-associated diseases [18]. In our study, the RANKL/OPG ratio was twofold higher in INS patients than in the patients of the reference group. Conversely, Ozkaya et al. found an increased OPG/RANKL ratio in children with chronic renal failure, which may represent, as suggested by the authors, a compensatory mechanism to the negative balance of bone remodeling in this disease [19].
The relationship between OPG and other markers of bone metabolism in patients with chronic glomerulonephritis treated with GCS was assessed by Sasaki et al. [20]. These authors observed a significant reduction in serum OPG immediately after starting the GCS treatment. This lack of a progressive decrease in serum OPG levels at consecutive examinations 1, 3, and 6 months post-treatment initiation could be due in part to the reduction in GCS dose. However, the limitation of that study was related to the low number of patients with a different original diagnosis.
Freundlich et al. [21] observed an up-regulation of RANKL in normal osteoblasts incubated with sera of NS children in relapse and normal expression in those incubated with sera of NS children in remission; this result suggests that changes in RANKL expression in NS children are transient and reversible. The study of Faienza et al. [22] showed a down-regulation of OPG and over-expression of RANKL in T-cells from patients with a 21-hydroxylase deficiency who were treated with GCS, compared with controls. Similarly increased concentrations of RANKL and decreased concentrations of OPG were found in the serum of these patients.
Opinions on BMD in INS children treated with GCS are equivocal. GCS have been reported to have much less of an adverse effect on bone in patients with non-inflammatory conditions than in the presence of inflammation [3, 4, 23]. The results of recent animal studies demonstrate that GCS administration during growth can result in decreased bone formation, decreased bone resorption, and a reduction in age-dependent increases in trabecular bone mineral and trabecular thickness [24, 25]. However, it is likely that the underlying disease also carries the risk of osteoporosis. In this setting, the independent effects of GCS on bone turnover and bone structure are not easily apparent. Leonard et al. [3] found a significant reduction in BMC in patients with Crohn’s disease, but a normal spine or even greater whole-body BMC in patients with steroid-sensitive NS. The explanation for this difference may be the detrimental effects on bone metabolism of inflammatory cytokines such as TNF alpha and interleukin-1 and -6, which are similar to GCS effects [26]. Childhood NS, in contrast to other systemic diseases, is associated with transient increases in cytokine levels, which resolves with GCS treatment and disease remission. Burnham et al. [23] suggested that inflammatory cytokines induced deleterious changes in osteoblast GCS metabolism.
We confirmed a significant positive correlation between CGCS and an increased serum RANKL concentration and increased RANKL/OPG ratio. The correlation with serum OPG was negative, but not statistically significant. A possible explanation for this result is the fact that GCS decrease the OPG level only during the therapy itself. We suggest that an increase in serum OPG levels post-GCS treatment could possibly compensate for an increase in bone resorption. Similarly, the OPG level was not correlated to the cumulative or current dose of glucocorticoids in patients after cardiac transplant [27]. The study of Asanuma et al. [28] even found a positive correlation between OPG and cumulative dose of steroids.
We found a statistically significant higher serum RANKL concentration and higher RANKL/OPG ratio in the group of children treated with a high dose of GCS in comparison to the group of children who had minimal exposure to GCS. In a further analysis, we found a higher serum level of RANKL and lower level of OPG in children receiving GCS; however, the difference between the levels of OPG was highly statistically significant and that between the levels of RANKL was borderline statistically significant. Freundlich et al. [21] reported that changes in osteoblastic RANKL expression were not influenced by the concurrent administration of steroids. Further analysis according to the actual dose or number of days of therapy goes beyond this paper.
High CGSC was independently associated with an increased serum RANKL concentration and an increased RANKL/OPG ratio, even when controlled for BMI Z-score. It is well known fact that complex hormonal changes occur in obesity, such as elevations in the concentration of insulin, sex hormone, and leptin, and that this may increase osteoblast activity and decrease osteoclast activity [29, 30].
There is a growing body of literature suggesting the role of factors other than GCS drugs in the pathogenesis of iathrogenic osteoporosis. CSA has been confirmed to be involved in the pathogenesis of post-transplant osteoporosis and vascular disease [31]. CSA, similar to GCS, has been shown to inhibit OPG and stimulate RANKL in cell cultures [32]. However, the results from clinical studies are equivocal, probably because in most protocols the CSA is used together with GCS, and the authors were consequently not able to exclude other important confounders, such as hormonal disturbances, vascular changes in the course of chronic kidney disease, low BMI, or decreased physical activity. We also were only able to present the increased relative risk of higher serum RANKL in NS children treated with CSA. However, our findings were not conclusive enough to suggest an influence of CSA treatment on serum RANKL because the samples were obtained from patients at different time-points during their treatment regimen. Further studies are necessary to confirm this observation.
Another issue is the correlation between the parameters analyzed and the BMD. Results from cross-sectional studies in humans vary from no association between BMD and OPG [33, 34] to a decrease in BMD with increasing OPG [35]. Similarly, for RANKL, a few studies have shown no association between RANKL and BMD [36], while others have shown a decrease in BMD with increasing RANKL levels [37]. The possible consequences of corticosteroid therapy on BMD in NS children have also been contradictory, resulting in low [38] or normal [4, 39] BMD values. The reason for these differences may be that the DXA examination was conducted in an optional time-frame, with no relation to disease activity and steroid administration. In our analysis, we assessed the BMC only in 24/40 children treated with high-dose GCS. In accordance with the new official position of the International Society for Clinical Densitometry on DXA evaluation in children and adolescents [40], we confirmed GIOP only in two of 24 children. We noted an inverse correlation with the prevailing RANKL levels. Longer follow-up studies with a larger number of patients will be needed to evaluate the impact of RANKL levels on the reduction in BMD.
In conclusion, we observed that: (1) INS children treated with GCS had an increased serum RANKL level, increased RANKL/OPG ratio, and decreased serum OPG level; (2) there was a significant positive correlation between the cumulative dose of GCS and the serum RANKL and RANKL/OPG ratio, but not with the serum OPG level; (3) concurrent GCS treatment increased the serum RANKL level and decreased the serum OPG level; (4) serum RANKL level, but not OPG level, correlated with the BMD Z-score, but further studies are necessary to confirm this observation.
Our study should be interpreted within the context of its limitations. First, we were not able to obtain sufficient samples from children suffering their first attack of INS before they had received GCS treatment to assess the influence of proinflammatory cytokines on serum RANKL and OPG levels. In a furture study, we also plan to monitor the level of both cytokines in the relapse of NS and then in the remission of proteinuria. Secondly, serum RANKL and OPG may not reflect the levels and activity of these cytokines in the bone microenvironment, since a small amount of locally acting cytokines leak to systemic circulation [41], and a part of serum OPG and RANKL may also originate from non-skeletal sources. Thirdly, the commercially available assays were designed to detect all forms of OPG (monomer, dimer, RANKL/OPG complex) and not exclusively the dimeric form, which is thought to be the biologically active form [41]. Fourthly, serum RANKL constitutes only a small part of total RANKL, as the majority is cell bound and thus not detectable in the circulation. However, a more accurate method that assesses cell-surface production of RANKL is very impractical in humans [41] because it requires bone biopsy.
References
Cushing H (1932) The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp 50:137–195
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Leonard MB, Zemel BS (2002) Current concepts in pediatric bone disease. Pediatr Clin North Am 49:143–173
Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA (2004) Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055
Kostenuik PJ, Shalhoub V (2001) Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des 7:613–635
Reichardt HM, Tuckermann JP, Bauer A, Schütz G (2000) Molecular genetic dissection of glucocorticoid receptor function in vivo. Z Rheumatol 59:1–5
Chiodini I, Carnevale V, Torlontano M, Fusilli S, Guglielmi G, Pileri M, Modoni S, Di Giorgio A, Liuzzi A, Minisola S, Cammisa M, Trischitta V, Scillitani A (1998) Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing's syndrome. J Clin Endocrinol Metab 83:1863–1867
Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140:4382–4389
Oelzner P, Franke S, Lehmann G, Eidner T, Müller A, Wolf G, Hein G (2007) Soluble receptor activator of NFkappa B-ligand and osteoprotegerin in rheumatoid arthritis-relationship with bone mineral density, disease activity and bone turnover. Clin Rheumatol 26:2127–2135
Turk N, Cukovic-Cavka S, Korsic M, Turk Z, Vucelic B (2009) Proinflammatory cytokines and receptor activator of nuclear factor kappaB-ligand/osteoprotegerin associated with bone deterioration in patients with Crohn's disease. Eur J Gastroenterol Hepatol 21:159–166
A report of the International Study of Kidney Disease in children (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephritic syndromefrom initial response to prednisone. J Pediatr 98:561–564
Wasilewska A, Rybi-Szuminska A, Zoch-Zwierz W (2009) Serum osteoprotegrin (OPG) and receptor activator of nuclear factor κB (RANKL) in healthy children and adolescents. J Pediatr Endocrinol Metab 22:1099–1104
Palczewska I, Niedźwiecka Z, Szilágyi-Pagowska I (2000) Secular growth trends in children and youth of Warsaw in the last twenty years. Med Wieku Rozwoj 4:161–176
Płudowski P, Matusik H, Olszaniecka M, Lebiedowski M, Lorenc RS (2005) Reference values for the indicators of skeletal and muscular status of healthy Polish children. J Clin Densitom 8:164–177
Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Töz H, Atilla G, Hughes FJ, Belibasakis GN (2007) Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol 34:370–376
Ozkaya O, Buyan N, Bideci A, Gonen S, Ortac E, Fidan K, Cinaz P, Söylemezoğlu O (2007) Osteoprotegerin and RANKL serum levels and their relationship with serum ghrelin in children with chronic renal failure and on dialysis. Nephron Clin Pract 105:c153–c158
Sasaki N, Kusano E, Ando Y, Nemoto J, Iimura O, Ito C, Takeda S, yano K, Tsuda E, Asano Y (2002) Changes in osteoprotegerin and markers of bone metabolism during glucocortcoid treatment in patients with chronic glomerulonephritis. Bone 30:853–858
Freundlich M, Alonzo E, Bellorin-Font E, Weisinger JR (2005) Increased Osteoblastic activity and expression of Receptor Activator if NF-kB Ligand in nonuremic Nephrotic Syndrome. J Am Soc Nephrol 16:2198–2204
Faienza MF, Brunetti G, Colucci S, Piacente S, Ciccarelli M, Giordani L, Giovanni C, D’Amore M, Albanese L, Cavallo L, Grano M (2009) J Clin Endocrinol Metab 94:2269–2276
Burnham JM, Shults J, Petit MA, Semeao E, Beck TJ, Zemel BS, Leonard MB (2007) Alterations in proximal femur geometry in children treated with glucocorticoids for Crohn disease or nephritic syndrome: impact of the underlying disease. J Bone Miner Res 22:551–559
Ikeda S, Morishita Y, Tsutsumi H, Ito M, Shiraishi A, Arita S, Akahoshi S, Narusawa K, Nakamura T (2003) Reductions in bone turnover, mineral, and structure associated with mechanical properties of lumbar vertebra and femur in glucocorticoid-treated growing minipigs. Bone 33:779–787
Ortoft G, Andreassen TT, Oxlund H (1999) Growth hormone increases cortical and cancellous bone mass in young growing rats with glucocorticoid-induced osteopenia. J Bone Miner Res 14:710–721
Rall LC, Roubenoff R (2004) Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 43:1219–1223
Fahrleitner A, Prenner G, Leb G, Tscheliessnigg KH, Piswanger-Sölkner C, Obermayer-Pietsch B, Portugaller HR, Berghold A, Dobnig H (2003) Serum osteoprotegerin is a major determinant of bone density development and prevalent vertebral fracture status following cardiac transplantation. Bone 32:96–106
Asanuma Y, Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, Raggi P, Sokka T, Pincus T, Stein CM (2007) Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis 195:135–141
Reid IR, Cornish J, Baldock PA (2006) Nutrition-related peptides and bone homeostasis. J Bone Miner Res 21:495–500
Smith JD, Al-Amri M, Sniderman AD, Cianflone K (2006) Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoA1 ratio in Asian Indian and Caucasian men and women. Nutr Metab 3:18–20
Luke RG (1994) New issues in therapy after renal transplantation. N Engl J Med 331:393–394
Hofbauer LC, Riggs BL, Dunstan CR, O’Brien T, Khosla S (1999) Cyclosporin A and glucocorticoids inhibit osteoprotegerin production in human osteoblastic and coronary artery smooth muscle cells: potential mechanism of post-transplantation osteoporosis and vascular disease. J Bone Miner Res 14:176–182
Abrahamsen B, Hjelmborg JV, Kostenuik P, Stilgren LS, Kyvik K, Adamu S, Brixen K, Langdahl BL (2005) Circulating amounts of osteoprotegerin and RANK ligand: genetic influence and relationship with BMD assessed in female twins. Bone 6:727–735
Uemura H, Yasui T, Miyatani Y, Yamada M, Hiyoshi M, Arisawa K, Irahara M (2008) Circulating profiles of osteoprotegerin and soluble receptor activator of nuclear factor kappaB ligand in post-menopausal women. J Endocrinol Investig 31:163–168
Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P (2005) Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 111:2461–2468
Kim JG, Kim JH, Lee DO, Kim H, Kim JY, Suh CS, Kim SH, Choi YM (2008) Changes in the serum levels of osteoprotegerin and soluble receptor activator for nuclear factor kappaB ligand after estrogen-progestogen therapy and their relationships with changes in bone mass in postmenopausal women. Menopause 15:357–362
Leonard MB (2007) Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics 119:S166–S174
Gulati S, Godbole M, Singh U, Gulati K, Srivastava A (2003) Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease? Am J Kidney Dis 41:1163–1169
Freundlich M (2006) Bone mineral content and mineral metabolism during cyclosporine treatment of nephrotic Syndrome. J Pediatr 149:383–389
Bianchi ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ (2010) Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 25:37–47
Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Pavlidou V, Koukoulis G, Avramidis A (2008) Acute changes in serum osteoprotegerin and receptor activator for nuclear factor-kappaB ligand levels in women with established osteoporosis treated with teriparatide. Eur J Endocrinol 158:411–425
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wasilewska, A., Rybi-Szuminska, A. & Zoch-Zwierz, W. Serum RANKL, osteoprotegerin (OPG), and RANKL/OPG ratio in nephrotic children. Pediatr Nephrol 25, 2067–2075 (2010). https://doi.org/10.1007/s00467-010-1583-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1583-1