Abstract
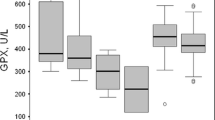
Increased lipid peroxidation (LP) has been observed in dialysis patients and in predialysis adults with advanced chronic renal failure (CRF). The aim of this study was to investigate whether predialysis CRF children have increased LP in plasma and red blood cells (RBC) and to evaluate the activity of the antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)] in RBC. Concentrations of selenium (Se), copper (Cu), and zinc (Zn)—cofactors of these enzymes—were determined both in erythrocytes and in plasma. LP was monitored by plasma and erythrocyte malonyldialdehyde (MDA) and by plasma organic hydroperoxide (OHP) concentrations. Forty-six predialysis children, aged 5–18 years, divided into two groups according to their serum creatinine levels [group I (n=14, mean serum creatinine 421.61±141.08 μmol/l), group II (n=32, mean serum creatinine 174.94±45.50 μmol/l)] and 27 age-matched healthy subjects were enrolled in the study. Significantly higher concentrations of plasma and erythrocyte MDA and plasma OHP, significantly lower activities of GSH-Px and CAT, and significantly lower concentrations of erythrocyte Se, Cu, and Zn and plasma Se and Cu were found in both groups of renal patients compared with controls. The SOD activity was reduced in both groups of CRF children. In group I the activity of SOD and GSH-Px was significantly lower than in group II. In summary, there is increased LP in plasma and RBC in children with predialysis CRF, even those patients with moderate renal insufficiency. The activity of the enzymatic antioxidant defense system is reduced in the RBC of predialysis patients. The antioxidant capacity is related to the severity of renal failure.
Similar content being viewed by others
References
Marx ER, Wicckens DG, Griffin JFK, Kyle P, Curtis JR, Dormandy TL (1987) Oxygen free radicals linked to many diseases. Science 2:529–531
Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK (2003) Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int 63:179–185
Yawata Y, Jacob H (1975) Abnormal red cell metabolism causing hemolysis in uremia: nature of the defect and its persistence despite adequate hemodialysis. Blood 45:231–239
Griendling KK, Sorecsu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Cir Res 86:494–501
Galli F, Ronco C (2000) Oxidant stress in hemodialysis. Nephron 84:1–5
Taccone-Gallucci M, Giardini O, Lubrano R, Mazarella V (1986) Red blood cell membrane lipid peroxidation in continuous ambulatory peritoneal dialysis patients. Am Clin Nephrol 6:92–95
Zwolińska D (1998) Lipid peroxidation and antioxidant enzymes in patients with chronic renal failure. Adv Clin Exp Med 7:87–92
Durak I, Akyol O, Basesme E, Canbolat O, Kavutcu M (1994) Reduced erythrocyte defence mechanisms against free radical toxicity in patients with chronic renal failure. Nephron 66:76–80
Mimic-Oka J, Simic T, Djukanovic L, Reljic Z, Davicevic Z (1999) Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin Nephrol 51:233–241
Richard MJ, Arnaud J, Jurkovitz C, Hachache T, Meftahi H, Laporte F, Foret M, Favier A, Cordonnier D (1991) Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron 57:10–15
Taccone-Gallucci M, Giardini O, Lubrano R, Bandino D, Mazzarella V, Mannarino O, Meloni C, Moroseti M, Elli M, Tozzo C, Strolighi L, Casciani CU (1987) Red blood cell lipid peroxidation in predialysis chronic renal failure. Clin Nephrol 27:238–241
Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thavenin M, Jaudon MC, Zingraff J, Verger C, Jungers P, Descamps-Latscha B (1996) Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med 21:845–853
Mimic-Oka J, Simic T, Ekmescic V, Dragicevic P (1995) Erythrocyte glutathione peroxidase and superoxide dismutase activities in different stages of chronic renal failure. Clin Nephrol 44:44–48
Asoyama K, Shiki Y, Ito H, Hasegowa O, Miyao A, Hayashibe H, Dobashi K, Kato K (1990) Antioxidant enzymes and lipoperoxide in blood in uremic children and adolescents. Free Radic Biol Med 9:105–109
Sommerburg O, Grune T, Ehrich JH, Siemens WG (2002) Adaptation of glutation-peroxidase activity to oxidative stress occurs in children but not in adult patients with end-stage renal failure undergoing hemodialysis. Clin Nephrol 58 [Suppl 1]:S31–S36
Turi S, Varga I, Matkovics B, Dobos E (1991) Erythrocyte defence mechanisms against free oxygen radicals in haemodialysed uraemic children. Pediatr Nephrol 5:179–183
Otting VU, Hellmann C (1990) Malondialdehydkonzentration (MDA) im Serum chronisch niereninsuffizienter, chronisch hämodialysierter und nierentransplantierter Kinder. Z Urol Nephrol 83:141–148
Chkawa H, Ohski N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Biochem 95:351–358
Nourooz-Zadeh J, Tajaddini Sarmadi J, Wolff S (1994) Measurement of plasma hydroperioxide concentration by ferrous oxidation xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–168
Aebi H (1974) Catalase, vol 2. In: Bergmeyer HU (eds) Methods of enzymatic analysis. Weinheim Academic Press, New York, pp 673–678
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Martin-Mateo MC, Sanchez-Portugal M, Iglesias S, Paula A de, Bustamante J (1999) Oxidative stress in chronic renal failure. Ren Fail 21:155–167
Trznadel K, Pawlicki L, Kędziora J, Luciak M, Błaszczyk J, Buczyński A (1989) Aktywność dysmutazy ponadtlenkowej w erytrocytach oraz stężenie malonylodialdehydu w erytrocytach i osoczu w różnych okresach niewydolności nerek. Pol Arch Med Wewn 80:261–266
Annuk M, Fellström B, Åkerblom O, Zilmer K, Vihalemm T, Zilmer M (2001) Oxidative stress markers in pre-uremic patients. Clin Nephrol 56:308–314
Zachara BA, Adamowicz A, Trafikowska U, Trafikowska A, Manitius J, Nartowicz E (2001) Selenium and glutathione levels and glutathione peroxidases and some other antioxidant parameters in blood of patients with chronic renal failure. J Trace Elem Med Biol 15:161–166
Dasgupta A, Hussain S, Ahmad S (1992) Increased lipid peroxidation in patients on maintenance hemodialysis. Nephron 60:56–59
Lucchi L, Banni S, Capelli B, Medici G, Melis MP, Tomasi A, Vannini V, Lusvarghi E (1993) Conjugated diene fatty acids in patients with chronic renal failure: evidence of increased lipid peroxidation? Nephron 65:401–409
Dursun E, Ozben T, Suleymanlar G, Dursun B, Yakupoglu G (2002) Effect of hemodialysis on the oxidative stress and antioxidants. Clin Chem Lab Med 40:1009–1013
Konukoglu D, Ercan M, Ayaz M, Onen S (2001) Plasma and erythrocytes antioxidant status and trace element levels in proteinuric patients with moderate glomerular function. J Trace Elem Med Biol 15:119–122
Nagase S, Aoyagi K, Hirayama A, Gotoh M, Ueda A, Tomida C, Kamezaki T, Nagai Y, Kikuchi H, Koyama A (1997) Favorable effect of hemodialysis on decreased serum antioxidant activity in hemodialysis patients demonstrated by electron spin resonance. J Am Soc Nephrol 8:1157–1163
Ozden M, Maral H, Akaydin D, Cetinalp P, Kalender B (2002) Erythrocyte glutathione peroxidase activity, plasma malonyldialdehyde and erythrocyte glutathione levels in hemodialysis and CAPD patients. Clin Biochem 35:269–273
Paul JL, Sall ND, Soni T, Poignet JL, Lindenbaum A, Man NK, Moatti N, Raichvarg D (1993) Lipid peroxidation abnormalities in hemodialyzed patients. Nephron 64:106–109
Vanella A, Geremia E, Pinturo R, Triolo P, Liuzzo G, Triolo C, Custorella A, Condorelli G, Giglio A (1983) Superoxide dismutase activity and reduced glutathione content in erythrocytes of uremic patients on chronic dialysis. Acta Haematol 70:312–315
Zachara BA, Adamowicz A, Trafikowska U, Trafikowska A, Manitius J, Nartowicz E (2001) Selenium and glutathione levels and glutathione peroxidase activities in blood component of uremic patients on hemodialysis supplemented with selenium and treated with erythropoietin. J Trace Elem Med Biol 15:201–208
Van Den Branden C, Ceyssens B, De Craemer D, De Bleser P, Hellemans K, Geerts A, Verbeelen D (2000) Antioxidant enzyme gene expression in rats with remnant kidney induced chronic renal failure. Exp Nephrol 8:91–96
Avissar N, Ornt DB, Yagil Y, Horovitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ (1994) Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol 266: C367–C375
Adamowicz A, Trafikowska U, Trafikowska A, Zachara BA, Manitius J (2002) Effect of erythropoietin on selected antioxidant parameters in blood of uremic patients on long-term hemodialysis. Med Sci Monit 8:CR202–205
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zwolińska, D., Grzeszczak, W., Kiliś-Pstrusińska, K. et al. Lipid peroxidation and antioxidant enzymes in children with chronic renal failure. Pediatr Nephrol 19, 888–892 (2004). https://doi.org/10.1007/s00467-004-1512-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1512-2