Abstract
Background
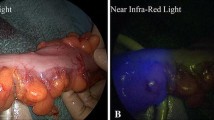
Decreased blood perfusion is an important risk factor for postoperative anastomotic leakage (AL). Fluorescence imaging with indocyanine green (ICG) provides a real-time assessment of intestinal perfusion. This study evaluated the utility of ICG fluorescence imaging in determining the transection line of the proximal colon during laparoscopic colorectal surgery with double stapling technique (DST) anastomosis.
Methods
This was a prospective single-institution study of 68 patients with left-sided colorectal cancers who underwent laparoscopic colorectal surgery between August 2013 and December 2014. After distal transection of the bowel, the specimen was extracted extracorporeally and then the mesentery was divided along the planned transection line determined by the surgeons’ judgement under normal q. After ICG was injected intravenously, intestinal perfusion of the proximal colon was assessed in the fluorescent imaging mode. Intestinal perfusion was examined in relation to the patient-, tumor- and surgery-related variables using univariate and multivariate analyses.
Results
ICG fluorescence imaging showed that intestinal perfusion was present at 3 mm (median) distal to the initially planned transection line. ICG fluorescence imaging resulted in a proximal change of the transection line by more than 5 mm in 18 patients (26.5 %) and, particularly, by more than 50 mm in 3 patients (4.4 %), compared with the initially planned transection line. Univariate analysis revealed that diabetes mellitus, anticoagulation therapy, preoperative chemotherapy and operative time were significantly associated with poor intestinal perfusion. Multivariate analysis identified anticoagulation therapy (P = 0.021) and preoperative chemotherapy (P = 0.019) as independent risk factors for poor intestinal perfusion. Three patients (4.5 %) with a change of transection line developed AL.
Conclusions
ICG fluorescence imaging is useful for determining the transection line in laparoscopic colorectal surgery with DST anastomosis. Anticoagulation therapy and preoperative chemotherapy are important risk factors for poor intestinal perfusion.


Similar content being viewed by others
References
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208:269–278
Branagan G, Finnis D, Wessex Colorectal Cancer Audit Working Group (2005) Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 48:1021–1026
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899
Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, Mills S, Stamos MJ (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148:65–71
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29:3608–3617
Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H (2013) Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 257:665–671
Kim JS, Cho SY, Min BS, Kim NK (2009) Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 209:694–701
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Sheridan WG, Lowndes RH, Young HL (1987) Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum 30:867–871
Kologlu M, Yorganci K, Renda N, Sayek I (2000) Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery 128:99–104
Nakayama S, Hasegawa S, Nagayama S, Kato S, Hida K, Tanaka E, Itami A, Kubo H, Sakai Y (2011) The importance of precompression time for secure stapling with a linear stapler. Surg Endosc 25:2382–2386
Nakayama S, Hasegawa S, Hida K, Kawada K, Sakai Y (2015) Obtaining secure stapling of a double stapling anastomosis. J Surg Res 193:652–657
Hasegawa S, Nakayama S, Hida K, Kawada K, Sakai Y (2015) Effect of tri-staple™ technology and slow firing on secure stapling using an endoscopic linear stapler. Dig Surg 32:353–360
Kawada K, Hasegawa S, Hida K, Hirai K, Okoshi K, Nomura A, Kawamura J, Nagayama S, Sakai Y (2014) Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc 28:2988–2995
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24:569–576
Urbanavicius L, Pattyn P, de Putte DV, Venskutonis D (2011) How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 3:59–69
Nachiappan S, Askari A, Currie A, Kennedy RH, Faiz O (2014) Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc 28:2513–2530
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 395:1025–1030
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27:3003–3008
Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA (2014) The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc 28:1695–1702
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220:82–92
Still J, Law E, Dawson J, Bracci S, Island T, Holtz J (1999) Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg 42:266–274
Waseda K, Ako J, Hasegawa T, Shimada Y, Ikeno F, Ishikawa T, Demura Y, Hatada K, Yock PG, Honda Y, Fitzgerald PJ, Takahashi M (2009) Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging 2:604–612
Spinoglio G, Priora F, Bianchi PP, Lucido FS, Licciardello A, Maglione V, Grosso F, Quarati R, Ravazzoni F, Lenti LM (2013) Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc 27:2156–2162
Sobin LH, Gospodarowicz MK, Wittekind Ch (eds) (2009) International Union Against Cancer (UICC) TNM Classification of Malignant Tumors, 7th edn. Wiley-Blackwell, West Sussex
Sakai Y, Kitano S (2015) Practice guidelines on endoscopic surgery for qualified surgeons by the endoscopic surgical skill qualification system. Asian J Endosc Surg 8:103–113
Hasegawa S, Nagayama S, Nomura A, Kawamura J, Sakai Y (2008) Multimedia article. Autonomic nerve-preserving total mesorectal excision in the laparoscopic era. Dis Colon Rectum 51:1279–1282
Kuroyanagi H, Oya M, Ueno M, Fujimoto Y, Yamaguchi T, Muto T (2008) Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surg Endosc 22:557–561
Foster ME, Brennan SS, Morgan A, Leaper DJ (1985) Colonic ischaemia and anastomotic healing. Eur Surg Res 17:133–139
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis 15:91–96
van Tonder JJ, Boon JM, Becker JH, van Schoor AN (2007) Anatomical considerations on Sudeck’s critical point and its relevance to colorectal surgery. Clin Anat 20:424–427
Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama M, Ishibe A, Watanabe K, Masui H, Nagahori K, Ichikawa Y, Endo I (2015) Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J Colorectal Dis 30:329–335
Kin C, Vo H, Welton L, Welton M (2015) Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 58:582–587
Miwa M (2010) The principle of ICG fluorescence mode. Open Surg Oncol J 2:26–28
Acknowledgments
The authors thank medical staffs and residents of Kyoto University Hospital gastrointestinal surgery for their participation in this study. We could not have completed the study without their diligence and support. This study was supported by a Grant from JFE (The Japanese Foundation for Research and Promotion of Endoscopy) (to K. Kawada). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kenji Kawada, Suguru Hasegawa, Toshiaki Wada, Ryo Takahashi, Shigeo Hisamori, Koya Hida and Yoshiharu Sakai have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Kawada, K., Hasegawa, S., Wada, T. et al. Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 31, 1061–1069 (2017). https://doi.org/10.1007/s00464-016-5064-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5064-x