Abstract
Background
Prolonged pneumoperitoneum has cerebral adverse effects that may delay recovery and cause postoperative cognitive changes. The purpose of this study was to investigate the effect of mannitol infusion after pneumoperitoneum initiation on cerebral oxygen balance and quality of postoperative recovery in patients undergoing prolonged retroperitoneal laparoscopy.
Methods
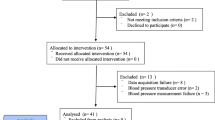
Forty patients scheduled for retroperitoneal laparoscopic radical excision of prostatic carcinoma were randomly divided into two groups (n = 20, each) to receive either 0.5 g/kg of 20% mannitol 150 min after the initiation of pneumoperitoneum or an equal volume of 0.9% normal saline. After surgery, time to extubation and recovery time were recorded. The Observer’s Assessment of Alertness/Sedation (OAA/S) scale was used to assess the quality of recovery. The Mini-Mental State Exam (MMSE) was given to test cognitive function preoperatively and at 1, 2, and 3 h after extubation. Blood samples from the jugular bulb and the radial artery were collected for blood gas analysis before CO2 insufflation and at 10, 60, and 180 min after insufflation.
Results
In the control group (without mannitol), the difference between arterial and venous oxygen content (CaO2–CvO2) before insufflation (6.21 ± 2.58 mL/dL) was significantly greater than it was 3 h after insufflation (2.63 ± 1.29 mL/dL; p < 0.05). Furthermore, 3 h after insufflation, the CaO2–CvO2 also was higher in the group that had been administered mannitol (5.93 ± 1.98 mL/dL) than it was in the control group at that time (p < 0.05). Lactic acid in both arterial and jugular venous blood of the control group at 3 h postinsufflation (2.39 ± 0.89 and 2.51 ± 0.72 mg/dL, respectively) had increased significantly from the preinsufflation values (1.18 ± 0.82 and 1.1 ± 0.85 mg/dL). In the group that received mannitol, the lactic acid levels 3 h postinsufflation were essentially the same as the preinsufflation values. The recovery and extubation times in those receiving mannitol (12.19 ± 2.12 and 20.14 ± 3.62 min, respectively) were significantly shorter than in the control group (21.25 ± 3.61 and 28.79 ± 4.73 min; p < 0.05). The OAAS scores of the mannitol group at the time of extubation and 10 min afterward was significantly higher than these scores in the control group (p < 0.05). One hour and 2 h after extubation, the cognitive function score of the mannitol group was significantly higher than for the control group (p < 0.05).
Conclusions
After prolonged retroperitoneal laparoscopy, there is an imbalance between oxygen supply and demand. A small dose of mannitol can effectively improve cerebral oxygen metabolism, recovery, and cognitive function after the operation.

Similar content being viewed by others
Abbreviations
- ASA:
-
American Society of Anesthesiology
- BIS:
-
Bispectral index
- CaCO2 :
-
Arterial carbon dioxide content
- CaO2 :
-
Arterial oxygen content
- CaO2–CvO2 :
-
Arteriovenous O2 content difference
- CBF:
-
Cerebral blood flow
- CEO:
-
Cerebral extraction of oxygen
- CjvCO2 :
-
Jugular venous carbon dioxide content
- CjvO2 :
-
Jugular venous oxygen content
- CMRO2 :
-
Cerebral metabolic rate of oxygen
- CvO2 :
-
Venous oxygen content
- Glca :
-
Arterial glucose
- Glcjv :
-
Jugular venous glucose
- Laca :
-
Arterial lactate
- Lacjv :
-
Jugular venous lactate
- MMSE:
-
Mini-mental state exam
- OAA/S:
-
Observer’s Assessment of Alertness/Sedation
- PaCO2 :
-
Arterial partial pressure carbon dioxide
- pHa :
-
Arterial pH
- pHjv :
-
Jugular venous pH
- PjvO2 :
-
Jugular venous partial pressure oxygen
- SaO2 :
-
Arterial oxygen saturation
- SjvO2 :
-
Jugular venous oxygen saturation
- TCCD:
-
Color-coded Doppler sonography
References
Park EY, Koo BN, Min KT, Nam SH (2009) The effect of pneumoperitoneum in the steep Trendelenburg position on cerebral oxygenation. Acta Anaesth Scand 53:895–899
Kurukahvecioglu O, Sare M, Karamercan A, Gunaydin B, Anadol Z, Tezel E (2008) Intermittent pneumatic sequential compression of the lower extremities restores the cerebral oxygen saturation during laparoscopic cholecystectomy. Surg Endosc Interv Tech 22:907–911
Tsypin LE, Mikhel’son VA, Chusov KP, Kazharskaia EI, Lazarev VV, Prokop’ev GG, Shchukin VV (2007) Central and cerebral hemodynamics during gynecological laparoscopic interventions in children. Anes Reanimatol 1:30–32
Lee J-R, Lee P-B, Do S-H, Jeon Y-T, Lee J-M, Hwang JY, Han S-H (2006) The effect of gynaecological laparoscopic surgery on cerebral oxygenation. J Int Med Res 34:531–536
Gipson CL, Johnson GA, Fisher R, Stewart A, Giles G, Johnson JO, Tobias JD (2006) Changes in cerebral oximetry during peritoneal insufflation for laparoscopic procedures. J Minim Access Surg 2:67–72
Moncure M, Salem R, Moncure K, Testaiuti M, Marburger R, Ye X, Brathwaite C, Ross SE (1999) Central nervous system metabolic and physiologic effects of laparoscopy. Am Surg 65:168–172
Lavinio A, Menon DK (2011) Intracranial pressure: why we monitor it, how to monitor it, what to do with the number and what’s the future? Curr Opin Anaesth 24:117–123
Streich B, Decailliot F, Perney C, Duvaldestin P (2003) Increased carbon dioxide absorption during retroperitoneal laparoscopy. Br J Anaesth 91:793–796
Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, Grimaud D, Leverve X (2009) Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med 35(3):471–479
Piyush U, Tripathi V, Singh R, Sachan D (2010) Role of hypertonic saline and mannitol in the management of raised intracranial pressure in children: a randomized comparative study. J Pediatr Neurosci 5(1):18–21
Sagsoz N, Kisa U, Apan A (2002) Ischaemia-reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod 17:2972–2976
Yilmaz N, Dulger H, Kiymaz N et al (2007) Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain Res 1164:132–135
Gillbe C, Sage F, Gutteridge J (1996) Mannitol: molecule magnifique or a case of radical misinterpretation [Commentary]. Free Radic Res 24(1):1–7
Luvisotto TL, Auer RN, Sutherland GR (1996) The effect of mannitol on experimental cerebral ischemia revisited. Neurosurgery 38(1):131
Size K, Wong E, Lum CM, Woo J (2000) Factors predicting stroke disability at discharge: a study of 793 Chinese. Arch Phys Med Rehabil 81(7):876–880
Zhuang X, Zeng Y, Chen B (2003) Modern anesthesiology, 3rd edn. People’s Health, Beijing, pp 1363–1388
Li L, Chen X (2002) The effect of carbon dioxide pneumoperitoneum on nervous system. Foreign Med Sci (Surg) 29(5):288–289
Miller RD, Miller S (2004) Anesthesia, 6th edn. Churchill Livingstone, New York, pp 2292–2299
Fernandez-Cruz L, Saenz A, Taura P, Benarroch G, Nies C, Astudillo E (1994) Pheochromocytoma: laparoscopic approach with CO2 and helium pneumoperitoneum. Endosc Surg Allied Technol 2:300–304
De Cosmo G, Lannace E, Primieri P, Valente MR, Proietti R, Matteis M, Silvestrini M (1999) Changes in cerebral hemodynamics during laparoscopic cholecystectomy. Neurol Res 21:658
Kitajima T, Shinohara M, Ogata H (1996) Cerebral oxygen metabolism measured by near-infrared laser spectroscopy during laparoscopic cholecystectomy with CO2 insufflation. Surg Laparosc Endosc 6:210
Xiuli M, Liping Z, Jianyu J (2005) Effects of retroperitoneal carbon dioxide insufflation on the balance of cerebral oxygen metabolism. Chin J Min Inv Surg 6:433
Shun-hou HE (2002) General anesthetics and cerebral oxygen balance. Foreign Med Sci 23(4):218–220
Randall MS, Daniel JC (2000) Cerebral monitoring: jugular venous oximetry. Anesth Analg 90:559–566
Levy B (2006) Lactale and shock slate: the metabolic view. Curr Opin Gril Care 12(4):315
Leverve XM, Mustafa I (2002) Lactate: a key metabolite in the intercellular metabolic interplay. Crit Care 6(4):284–285
Nilsson OG, Brandt L, Ungerstedt U, Säveland H (1999) Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery 5:1176–1185
de Tournay-Jetté E, Dupuis G, Bherer L, Deschamps A, Cartier R, Denault A (2011) The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 25(1):95–104 Epub 2010 Jul 22
Kadoi Y, Saito S, Takahashi K, Fujita N, Goto F (2004) Jugular venous oxygen saturation during mild hypothermic versus normothermic cardiopulmonary bypass in elderly patients. Surg Today 34(5):399–404
Suehiro K, Okutai R (2011) Duration of cerebral desaturation time during single-lung ventilation correlates with mini mental state examination score. J Anesth 25(3):345–349
Burkhart CS, Rossi A, Dell-Kuster S, Gamberini M, Möckli A, Siegemund M, Czosnyka M, Strebel SP, Steiner LA (2011) Effect of age on intraoperative cerebrovascular autoregulation and near-infrared spectroscopy-derived cerebral oxygenation. Br J Anaesth 107(5):742–748
Morimoto Y, Yoshimura M, Utada K, Setoyama K, Matsumoto M, Sakabe T (2009) Prediction of postoperative delirium after abdominal surgery in the elderly. J Anesth 23(1):51–56
Tang L, Kazan R, Taddei R, Zaouter C, Cyr S, Hemmerling TM (2012) Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth 108(4):623–629
Bereczki D, Mihálka L, Szatmári S, Fekete K, Cesar DD, Fülesdi B, Csiba L, Fekete I (2003) Mannitol use in acute stroke: case fatality at 30 days and 1 year. Stroke 34:1730–1735
Zhao Z, Shao F (1999) The use of mannitol in acute cerebrovascular disease and points for attention. Chinese J New Drugs Clin Rem 18(2):l11–l15
Perez RS, Praqt E, Geurts J, Zuurmond WW, Patijn J, van Kleef M (2008) Treatment of patients with complex regional pain syndrome type I with mannitol: a prospective, randomized, placebo-controlled, double-blinded study. J Pain 9(8):678–686
Tsai SF, Shu KH (2010) Mannitol-induced acute renal failure. Clin Nephrol 74:70–73
Pérez-Pérez AJ, Pazos B, Sobrado J, Gonzalez L, Gándara A (2002) Acute renal failure following massive mannitol infusion. Am J Nephrol 22(5–6):573–575
Kalita J, Misra UK, Ranjan P, Pradhan PK, Das BK (2004) Effect of mannitol on regional cerebral blood flow in patients with intracerebral hemorrhage. J Neurol Sci 224:19–22
Sakowitz OW, Stover JF, Sarrafzadeh AS, Unterberg AW, Kiening KL (2007) Effects of mannitol bolus administration on intracranial pressure, cerebral extracellular metabolites, and tissue oxygenation in severely head-injured patients. J Trauma 62:292–298
Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, Jacquot C, Payen JF (2008) Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med 36:795–800
Luvisotto TL, Auer RN, Sutherland GR (1996) The effect of mannitol on experimental cerebral ischemia, revisited. Neurosurgery 38:131–139
Disclosures
Drs. Xiang Zhou, Ming-chun Wu, Yan-lin Wang, Xiao-yang Song, Na-jia Ling, Jun-zhe Yang, Dan Zhang, Bi-xi Li, and Jun Tao have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiang Zhou and Ming-chun Wu contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Zhou, X., Wu, Mc., Wang, Yl. et al. Mannitol improves cerebral oxygen content and postoperative recovery after prolonged retroperitoneal laparoscopy. Surg Endosc 27, 1166–1171 (2013). https://doi.org/10.1007/s00464-012-2569-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2569-9