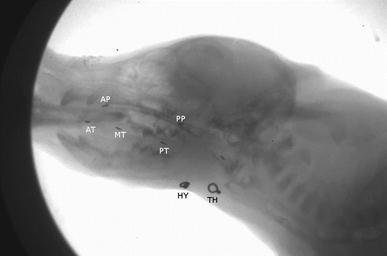
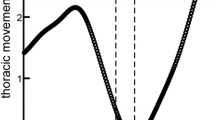
Abstract
The superior laryngeal nerve provides detailed sensory information from the mucosal surfaces of laryngeal structures superior to the vocal folds, including the valleculae. Injury to this nerve results in airway penetration and aspiration. Furthermore, such injuries might have an impact on the function of multiple structures involved in intraoral transport and swallowing due to connections within the brainstem. We sought to determine the effects of a surgical lesion of the superior laryngeal nerve on kinematics of the tongue, hyoid, and epiglottis during swallowing. We implanted radio-opaque markers into five infant pigs under anesthesia. Then we fed milk mixed with contrast agent to the pigs while they were recorded via video fluoroscopy, before and after a surgery to transect the superior laryngeal nerve. We digitized and rated airway protection in 177 swallows. We found that in most animals, swallow duration was shorter after nerve lesion. The hyoid also traveled a shorter distance after lesion. Frequently, individuals reacted differently to the same nerve lesion. We suggest that these differences are due to individual differences in neurological connections. When comparing hyoid kinematics between swallows with successful or failed airway protection, we found more consistency among individuals. This indicates that protecting the airway requires specific sets of kinematic events to occur, regardless of the neurological differences among individuals.





Similar content being viewed by others
References
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69.
Tsujimura T, Tsuji K, Ariyasinghe S, Fukuhara T, Yamada A, Hayashi H, Nakamura Y, Iwata K, Inoue M. Differential involvement of two cortical masticatory areas in modulations of the swallowing reflex in rats. Neurosci Lett. 2012;528:159–64.
Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25:323–33.
Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, Thexton AJ, German RZ. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia. 2013;28:404–12.
Bautista TG, Sun Q-J, Pilowsky PM. The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Prog Brain Res. 2014;212:253–75.
Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, Waranch DR, German RZ. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs. Laryngoscope. 2014;124:436–45.
Li Q, Minagi Y, Hori K, Kondo J, Fujiwara S, Tamine K, Inoue M, Maeda Y, Chen Y, Ono T. Coordination in oro-pharyngeal biomechanics during swallowing. Physiol Behav. 2015;147:300–5.
Gould FDH, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, German RZ. The physiologic impact of unilateral recurrent laryngeal nerve (RLN) lesion on infant oropharyngeal and esophageal performance. Dysphagia. 2015;30:714–22.
Gould FDH, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, German RZ. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol. 2016;120:495–502.
Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia. 2017;32:362–73.
Gross A, Ohlemacher J, German R, Gould F. LVC timing in infant pig swallowing and the effect of safe swallowing. Dysphagia. 2018;33:51–62.
Sulica L. The superior laryngeal nerve: function and dysfunction. Otolaryngol Clin N Am. 2004;37:193–201.
Hydman J, Mattsson P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve. 2008;38:1280–9.
Naidu L, Lazarus L, Partab P, Satyapal KS. Laryngeal nerve “anastomoses”. Folia Morphol. 2014;73:30–6.
Paskhover B, Wadie M, Sasaki CT. Thyroarytenoid cross-innervation by the external branch of the superior laryngeal nerve in the porcine model. Laryngoscope. 2014;125:177–9.
Iimura K, Suzuki H, Hotta H. Thyroxin and calcitonin secretion into thyroid venous blood is regulated by pharyngeal mechanical stimulation in anesthetized rats. J Physiol Sci. 2019;69:749–56.
Takahashi K, Shingai T, Saito I, Yamamura K, Yamada Y, Kitagawa J. Facilitation of the swallowing reflex with bilateral afferent input from the superior laryngeal nerve. Neurosci Lett. 2014;562:50–3.
Sasaki CT, Hundal JS, Kim Y-H. Protective glottic closure: biomechanical effects of selective laryngeal denervation. Ann Otol Rhinol Laryngol. 2005;114:271–5.
Suzuki T, Yoshihara M, Sakai S, Tsuji K, Nagoya K, Magara J, Tsujimura T, Inoue M. Effect of peripherally and cortically evoked swallows on jaw reflex responses in anesthetized rabbits. Brain Res. 2018;1694:19–28.
Wasserman JM, Sundaram K, Alfonso AE, Rosenfeld RM, Har-El G. Determination of the function of the internal branch of the superior laryngeal nerve after thyroidectomy. Head Neck. 2007;30:21–7.
Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg. 2014;22:439–43.
Folk D, Paskhover B, Wadie M, Wahba B, Sasaki CT. External branch of the superior laryngeal nerve mediated glottic closing force in the porcine model. Annal Otol Rhinol Laryngol. 2016;125:421–4.
Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550:287–304.
Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. Ann Otol Laryngol. 2002;111:291–4.
German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool. 1992;261:322–30.
German RZ, Crompton AW, Gould FDH, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia. 2017;32:73–7.
Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. Laryngoscope. 2013;123:1941–7.
Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, Lukasik SL, German RZ. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia. 2013;28:178–87.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Thexton AJ, Crompton AW, German RZ. Transition from sucking to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–43.
Neter J, Kutner M, Wasserman W, Nachtscheim C. Applied linear statistical models. New York: McGraw-Hill/Irwin; 1996.
DeLozier KR, Gould FDH, Ohlemacher J, Thexton AJ, German RZ. Impact of recurrent laryngeal nerve lesion on oropharyngeal muscle activity and sensorimotor integration in an infant pig model. J Appl Physiol. 2018;125:159–66.
Old MO, Oh SS, Feldman E, Hogikyan ND. Novel model to assess laryngeal function, innervation, and reinnervation. Ann Otol Rhinol Laryngol. 2011;120:331–8.
Peng ZW, Zu T, He QH, Shi CZ, Wei Z, Miao GD, Jing J, Lim KO, Zuo XN, Chan RC. Default network connectivity as a vulnerability marker for obsessive compulsive disorder. Psych Med. 2014;44:1475–84.
Wandschneider B, Hong SJ, Bernhardt BC, Fadaie F, Vollmar C, Koepp MJ, Bernasconi N, Bernasconi A. Developmental MRI markers cosegregate juvenile patients with myoclonic epilepsy and their healthy siblings. Neurology. 2019;93:e1272–e12801280.
Nagy A, Molfenter SM, Péladeau-Pigeon M, Stokely S, Stelle CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. BioMed Res Int 2014; Article ID 738971.
Nagy A, Molfenter SM, Péladeau-Pigeon M, Stokely S, Stelle CM. The effect of bolus consistency on hyoid velocity in healthy swallowing. Dysphagia. 2015;30:445–51.
Curtis J, Langenstein J, Schneider S. Superior and anterior hyoid displacement during swallowing in non-dysphagic individuals. Dysphagia. 2018;33:602–9.
Ueda N, Nohara K, Kotani Y, Tanaka N, Okuno K, Sakai T. Effects of the bolus volume on hyoid movements in normal individuals. J Oral Rehab. 2013;40:491–9.
McCullough GH, Kim Y. Effects of the Mendelsohn maneuver on extent of hyoid movement and UES opening post-stroke. Dysphagia. 2013;28:511–9.
Azola AM, Greene LR, Taylor-Kamara I, Macrae P, Anderson C, Humbert IA. The relationship between submental surface electromyography and hyo-laryngeal kinematic measures of Mendelsohn maneuver duration. J Speech Lang Hear Res. 2015;58:1627–36.
Inamoto Y, Saitoh E, Ito Y, Kagaya H, Aoyagi Y, Shibata S, Ota K, Fujii N, Palmer JB. The Mendelsohn Maneuver and its effects on swallowing: kinematic analysis in three dimensions using dynamic area detector CT. Dysphagia. 2018;33:419–30.
Pascual-Font A, Maranillo E, Merchán A, Vázquez T, Sañudo JR, Valderrama-Canales F. Proyecciones centrales del nervio laríngeo superior de la rata. Acta Otorr Esp. 2006;57:295–9.
Samson N, Praud J-P, Quenet B, Similowski T, Straus C. New insights into sucking, swallowing and breathing central generators: a complexity analysis of rhythmic motor behaviors. Neurosci Lett. 2017;683:90–5.
Acknowledgements
We thank Francois Gould, Christopher Mayerl, and anonymous reviewers for helpful comments on the manuscript, and Elaine Godec for assistance with processing data. This work was supported by the National Institutes of Health (DC9980 to RZG).
Funding
This study was funded by the National Institutes of Health (Grant Number DC9980).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors ARL, SA, and PD declare that they have no conflicts of interest. Author RZG received a research grant from the National Institutes of Health.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lammers, A.R., Abid, S., Ding, P. et al. Effects of Superior Laryngeal Nerve Lesion on Kinematics of Swallowing and Airway Protection in an Infant Pig Model. Dysphagia 35, 907–917 (2020). https://doi.org/10.1007/s00455-020-10100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10100-7