Abstract
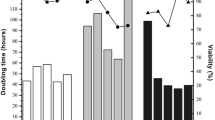
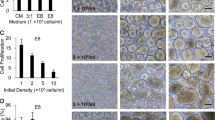
Human cell lines have attracted great interest because they are capable of producing glycosylated proteins that are more similar to native human proteins, thereby reducing the potential for immune responses. However, these cells have not been extensively characterized and cultured under serum-free suspension conditions. In this work, we describe the adaptation, growth, and cryopreservation of the human cell lines SK-Hep-1, HepG2, and HKB-11 under serum-free suspension conditions. The results showed that both HKB-11 and SK-Hep-1 adapted to serum-free suspension cultures in FreeStyle and SFM II, respectively. Kinetic characterization showed that the HKB-11 and SK-Hep-1 cells reached cell densities as high as 8.6 × 106 and 1.9 × 106 cells/mL, respectively. The maximum specific growth rates (μ max) were similar for both cells (0.0159/h for HKB-11 and 0.0186/h for SK-Hep-1). The growth limitation of adapted cells does not appear to be associated with glucose or glutamine depletion, nor with the formation of lactate in inhibitory concentrations. However, in both cases, ammonia production reached concentrations that are considered inhibitory to mammalian cells (2–5 mM). The adapted cells were also successfully cryopreserved under serum-free formulations. The SK-HEP-1 and HKB-11 cells that were adapted to serum-free suspension conditions might be suitable for use in the manufacturing of recombinant proteins, thereby eliminating the potential for the introduction of adventitious process contamination and greatly simplifying downstream protein purification.









Similar content being viewed by others
References
Walsh G (2002) Biopharmaceuticals and biotechnology medicines: an issue of nomenclature. Eur J Pharm Sci 15:135–138
Durocher Y, Butler M (2009) Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol 20:700–707
Berlec A, Strukelj B (2013) Current state and recent advances in biopharmaceutical production in Escherichia coli, yeasts and mammalian cells. J Ind Microbiol Biotechnol 40:257–274
Rader RA (2013) FDA biopharmaceutical product approvals and trends in 2012. BioProcess Intl 11:18–27
Rader RA (2013) An analysis of the US biosimilars development pipeline and likely market evolution. Bioprocess Intl 11:16–23
Grillberger L, Kreil TR, Nasr S, Reiter M (2009) Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J 4:186–201
Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A (2012) Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev 28:147–176
Pau MG, Ophorst C, Koldijk MH, Schouten G, Mehtali M, Uytdehaag F (2001) The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine 19:2716–2721
Jones D, Kroos N, Anema R, van Montfort B, Vooys A, van der Kraats S, van der Helm E, Smits S, Schouten J, Brouwer K, Lagerwerf F, van Berkel P, Opstelten D, Logtenberg T, Boutet A (2003) High-level expression of recombinant IgG in the human cell line PER.C6. Biotechnol Prog 19:163–168
Berdichevsky M, Gentile M, Hughes B, Meis P, Peltier J, Blumentals I, Auninus J, Altaras NE (2008) Establishment of higher passage PER.C6 cells for adenovirus manufacture. Biotechnol Prog 24:158–165
Tchoudakova A, Hensel F, Murillo A, Eng B, Foley M, Smith L, Schoenen F, Hildebrand A, Kelter A-R, Ilag LL, Vollmers HP, Brandlein S, McIninch J, Chon J, Lee G, Cacciuttolo M (2009) High level expression of functional human IgMs in human PER.C6® cells. MAbs 1:163–171
Kruif J, Kramer A, Nijhuis R, Zande V, Blanken R, Clements C, Visser T, Keehnen R, Hartog M, Throsby M, Logtenberg T (2010) Generation of stable cell clones expressing mixtures of human antibodies. Biotechnol Bioeng 106:741–750
Ross D, Brown T, Harper R, Pamarthi M, Nixon J, Bromirski J, Li CM, Ghali R, Xie H, Medvedeff G, Li H, Scuderi P, Arora V, Hunt J, Barnett T (2012) Production and characterization of a novel human recombinant alpha-1-antitrypsin in PER.C6 cells. J Biotechnol 162:262–273
Sanders BP, Edo-Matas D, Custers JHHV, Koldijk MH, Klaren V, Turk M, Luitjens A, Bakker WAM, Uytdehaag F, Goudsmit J, Lewis JA, Schuitemaker H (2013) PER.C6® cells as a serum-free suspension cell platform for the production of high titer poliovirus: a potential low cost of goods option for world supply of inactivated poliovirus vaccine. Vaccine 31:850–856
Fallaux FJ, Bout A, Van der Velde I, Van den Wollenberg DJM, Hehir KM, Keegan J, Auger C, Cramer SJ, Van Ormondt H, Van Der Eb AJ, Valerio D, Hoeben RC (1998) New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9:1909–1917
Yallop C, Crowley J, Cote J, Hegmans-Brouwer K, Lagerwerf F, Gagne R, Martin JC, Oosterhuis N, Opstelten DJ, Bout A (2008) PER.C6® cells for the manufacture of biopharmaceutical proteins. In: Knablein J (ed) Modern biopharmaceuticals: design, development and optimization, 1st edn. Wiley-VCH, Weinheim
Coco-Martin JM, Harmsen MM (2008) A review of therapeutic protein expression by mammalian cells. Bioprocess Intl 6:28–33
Langer E (2009) On the horizon: new expression systems to become common industry platforms. Biopharm Intl 22:2–4
Swiech K, Picanço-Castro V, Covas DT (2012) Human cells: new platform for recombinant therapeutic protein production. Protein Expr Purif 84:147–153
Moran N (2010) Shire’s replacement enzymes validate gene activation. Nat Biotechnol 28:1139–1140
Cho MS, Yee H, Chan S (2002) Establishment of a human somatic hybrid cell line for recombinant protein production. J Biomed Sci 9:631–638
Mei B, Chen Y, Chen J, Pan CQ, Murphy JE (2006) Expression of human coagulation factor VIII in a human hybrid cell line, HKB-11. Mol Biotechnol 34:165–178
Cho MS, Yee H, Brown C, Jeang K, Chan S (2001) An oriP expression vector containing the HIV-1 Tat/TAR transactivation axis produces high levels of protein expression in mammalian cells. Cytotechnology 37:23–30
Fischer S, Charara N, Gerber Am Wolfel J, Schiedner G, Voedisch B, Geisse S (2012) Transient recombinant protein expression in a human amniocyte cell line: the CAP-T® cell system. Biotechnol Bioeng 109:2250–2261
Tang L, Leong L, Sim D, Ho E, Gu J-M, Schneider D, Feldman RI, Monteclaro F, Jiang H, Murphy JE (2013) von Willebrand factor contributes to longer half-life of PEGylated factor VIII in vivo. Haemophilia 19:539–545
Turner BM, Turner VS (1980) Secretion of alpha-antitrypsin by an established human hepatoma cell line and by human/mouse hybrids. Somatic Cell Genet 6:1–14
Herlitschka SE, Schlokat U, Falkner FG, Dorne F (1998) High expression of a B-domain deleted factor VIII gene in a human hepatic cell line. J Biotechnol 61:165–173
Picanço-Castro V, Heinz S, Bott D, Behrmann M, Covas DT, Seifried E, Tonn T (2007) Recombinant expression of coagulation factor VIII in hepatic and non-hepatic cell lines stably transduced with third generation lentiviral vectors comprising the minimal factor VIII promoter. Cytotherapy 9:785–794
Campos-da-Paz M, Costa CS, Quilici LS, Simões IC, Kyaw CM, Maranhão AQ, Brigido MM (2008) Production of recombinant human factor VIII in different cell lines and the effect of human XBP1 co-expression. Mol Biotechnol 39:155–158
Kim EJ, Kim NS, Lee GM (1999) Development of a serum-free medium for dihydrofolate reductase-deficient Chinese hamster ovary cells (DG44) using a statistical design: beneficial effect of weaning of cells. In Vitro Cell Dev Biol Anim 35:178–182
Sinacore MS, Drapeau D, Adamson SR (2000) Adaptation of mammalian cells to growth in serum-free media. Mol Biotechnol 15:249–257
Schröder M, Matischak K, Friedl P (2004) Serum- and protein-free media formulations for the Chinese hamster ovary cell line DUKXB11. J Biotechnol 108:279–292
van der Valk J, Brunner D, De Smet K, Svenningsen ÅF, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G (2010) Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 24:1053–1063
Jaluria P, Konstantopoulos K, Betenbaugh M, Shiloach J (2008) Egr1 and Gas6 facilitate the adaptation of HEK-293 cells to serum-free media by conferring enhanced viability and higher growth rates. Biotechnol Bioeng 99:1443–1452
Price PJ, Gregory EA (1982) Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro 18:576–584
Gstraunthaler G (2003) Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex 20:275–281
Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G (2010) Serum-free cell culture: the serum-free media interactive online database. Altex 27:53–62
Hutchings SE, Sato GH (1978) Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc Natl Acad Sci USA 75:901–904
Hernández YG, Fischer RW (2007) Serum-free culturing of mammalian cells—adaptation to and cryopreservation in fully defined media. Altex 24:110–116
Muller N, Girard P, Hacker DL, Jordan M, Wurm FM (2005) Orbital shaker technology for the cultivation of mammalian cells in suspension. Biotechnol Bioeng 89:400–406
Maranga L, Aunins JG, Zhou W (2005) Characterization of changes in PER.C6TM cellular metabolism during growth and propagation of a replication-deficient adenovirus vector. Biotechnol Bioeng 90:645–655
Sun X, Goh PE, Wong KTK, Mori T, Yap MGS (2006) Enhancement of transient gene expression by fed-batch culture of HEK 293 EBNA1 cells in suspension. Biotechnol Lett 28:843–848
Loignon M, Perret S, Kelly J, Boulais D, Cass B, Bisson L, Afkhamizarreh F, Durocher Y (2008) Stable high volumetric production of glycosylated human recombinant IFNalpha2b in HEK293 cells. BMC Biotechnol 8:1–16
Altamirano C, Gòdia F, Cairó J (2008) Metabolismo de células de mamíferos cultivadas in vitro. In: Moraes AM, Augusto EFP, Castilho LR (eds) Tecnologia de cultivo de células animais: de biofármacos a terapia gênica, 1st edn. Roca, São Paulo
Moretti P, Behr L, Walter JG, Kasper C, Stahl F, Scheper T (2010) Characterization and improvement of cell line performance via flow cytometry and cell sorting. Eng Life Sci 10:130–138
Lifetechnologies (2014) GlutaMAX™ vs. glutamine. http://www.lifetechnologies.com/br/en/home/life-science/cell-culture/mammalian-cell-culture/media-supplements/glutamax-media/glutamax-vs-glutamine.html. Accessed 2 Feb 2014
Altamirano C, Berrios J, Vergara M, Becerra S (2013) Advances in improving mammalian cells metabolism for recombinant protein production. E J Biotechnol 16:1–14
Haldankar R, Kopchick JJ, Ridgway D (1999) Stable production of a human growth hormone antagonist from CHO cells adapted to serum-free suspension culture. Biotechnol Prog 15:336–346
Wong VVT, Ho KW, Yap MGS (2004) Evaluation of insulin-mimetic trace metals as insulin replacements in mammalian cell cultures. Cytotechnology 45:107–115
von Fircks S, Elmer R, Reiter M (2010) Method of producing serum-free insulin-free factor VII. US Patent 2010/0120093 A1, 13 May 2010
Acknowledgments
The authors acknowledge financial support from FAPESP (Process numbers 2012/04629-8 and 2012/02109-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biaggio, R.T., Abreu-Neto, M., Covas, D.T. et al. Serum-free suspension culturing of human cells: adaptation, growth, and cryopreservation. Bioprocess Biosyst Eng 38, 1495–1507 (2015). https://doi.org/10.1007/s00449-015-1392-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1392-9