Abstract
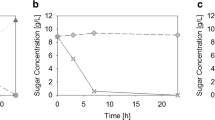
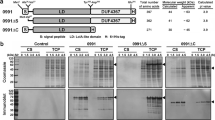
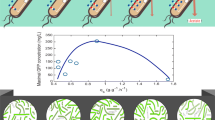
The secretion of recombinant proteins into the extracellular space by Escherichia coli presents advantages like easier purification and protection from proteolytic degradation. The controlled co-expression of a bacteriocin release protein aids in moving periplasmic proteins through the outer membrane. Since such systems have rarely been applied in continuous culture it seemed to be attractive to study the interplay between growth-phase regulated promoters controlling release protein genes and the productivity of a chemostat process. To avoid the use of antibiotics and render this process more sustainable, alternative plasmid selection mechanisms were required. In the current study, the strain E. coli JM109 harboring plasmid p582 was shown to stably express and secrete recombinant β-glucanase in continuous culture using a minimal medium. The segregational instability of the plasmid in the absence of antibiotic selection pressure was demonstrated. The leuB gene, crucial in the leucine biosynthetic pathway, was cloned onto plasmid p582 and the new construct transformed into an E. coli Keio (ΔleuB) knockout strain. The ability of the construct to complement the leucine auxotrophy was initially tested in shake-flasks and batch cultivation. Later, this strain was successfully grown for more than 200 h in a chemostat and was found to be able to express the recombinant protein. Significantly, it showed a stable maintenance of the recombinant plasmid in the absence of any antibiotics. The plasmid stability in a continuously cultivated E. coli fermentation, in the absence of antibiotics, with extracellular secretion of recombinant protein provides an interesting model for further improvements.











Similar content being viewed by others
Abbreviations
- t :
-
Batch/fed-batch/chemostat operating time, h
- X :
-
Dry biomass concentration, g L−1
- OD 600 :
-
Optical density
- S Gly :
-
Residual glycerol concentration in medium, g L−1
- E :
-
Recombinant β-glucanase activity in extracellular medium, kU mL−1
- c etPr :
-
Total protein concentration in extracellular medium, g L−1
- S AS :
-
Residual ammonium sulphate concentration in medium, g L−1
- c nPl :
-
Eluted plasmid concentration normalized to optical density of sample, ng μL−1
- L V,E :
-
Volumetric recombinant β-glucanase productivity, kU L−1 h−1
- D :
-
Space velocity during chemostat operation, h−1
References
Ni Y, Chen R (2009) Extracellular recombinant protein production from Escherichia coli. Biotechnol Lett 31:1661–1670
Hoskisson P, Hobbs G (2005) Continuous culture–making a comeback? Microbiol 151:3153–3159
Mashego MR, Rumbold K, De Mey M, Vandamme E, Soetaert W, Heijnen JJ (2007) Microbial metabolomics: past, present, and future methodologies. Biotechnol Lett 29:1–16
Vaiphei ST, Pandey G, Mukherjee KJ (2009) Kinetic studies of recombinant human interferon-gamma expression in continuous cultures of E. coli. J Ind Microbiol Biotechnol 36:1453–1458
Rodríguez-Carmona E, Cano-Garrido O, Dragosits M, Maurer M, Mader A, Kunert R, Mattanovich D, Villaverde A, Vázquez F (2012) Recombinant fab expression and secretion in Escherichia coli continuous culture at medium cell densities: influence of temperature. Process Biochem 47:446–452
Miksch G, Fiedler E, Dobrowolski P, Friehs K (1997) The kil gene of the ColE1 plasmid of Escherichia coli controlled by a growth-phase-dependent promoter mediates the secretion of a heterologous periplasmic protein during the stationary phase. Arch Microbiol 167:143–150
Robbens J, Raeymaekers A, Steidler L, Fiers W, Remaut E (1995) Production of soluble and active recombinant murine interleukin-2 in Escherichia coli: high level expression, kil-induced release and purification. Protein Expr Purif 6:481–486
Lin WJ, Huang SW, Chou CP (2001) High-level extracellular production of penicillin acylase by genetic engineering of Escherichia coli. J Chem Technol Biotechnol 76:1030–1037
Wal F, Hagen-Jongman C, Oudega B (1995) Optimization of bacteriocin-release-protein-induced protein release by Escherichia coli: extracellular production of the periplasmic molecular chaperone FaeE. Appl Microbiol Biotechnol 44:459–465
Beshay U, Miksch G, Friehs K, Flaschel E (2007) Increasing the secretion ability of the kil gene for recombinant proteins in Escherichia coli by using a strong stationary-phase promoter. Biotechnol Lett 29:1893–1901
Beshay U, Miksch G, Friehs K, Flaschel E (2009) Integrated bioprocess for the production and purification of recombinant proteins by affinity chromatography in Escherichia coli. Bioprocess Biosyst Eng 32:149–158
Borriss R, Olsen O, Thomsen KK, von Wettstein D (1989) Hybrid bacillus endo-(1-3,1-4)-beta-glucanases: construction of recombinant genes and molecular properties of the gene products. Carlsberg Res Commun 54:41–54
Celestino KRS, Cunha RB, Felix CR (2006) Characterization of a beta-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry. BMC Biochem. doi:10.1186/1471-2091-7-23
Boyce A, Walsh G (2007) Production, purification and application-relevant characterisation of an endo-1,3(4)-β-glucanase from Rhizomucor miehei. Appl Microbiol Biotechnol 76:835–841
Gil N, Gil C, Amaral ME, Costa AP, Duarte AP (2009) Use of enzymes to improve the refining of a bleached Eucalyptus globulus kraft pulp. Biochem Eng J 46:89–95
Hakamada Y, Hatada Y, Ozawa T, Ozaki K, Kobayashi T, Ito S (2001) Identification of thermo stabilizing residues in a Bacillus alkaline cellulase by construction of chimeras from mesophilic and thermostable enzymes and site-directed mutagenesis. FEMS Microbiol Lett 195:67–72
Kroll J, Klinter S, Schneider C, Voss I, Steinbüchel A (2010) Plasmid addiction systems: perspectives and applications in biotechnology. Microb Biotechnol 3:634–657
Friehs K (2004) Plasmid copy number and plasmid stability. Adv Biochem Eng Biotechnol 86:47–82
Kroll J, Steinle A, Reichelt R, Ewering C, Steinbüchel A (2009) Establishment of a novel anabolism-based addiction system with an artificially introduced mevalonate pathway: complete stabilization of plasmids as universal application in white biotechnology. Metab Eng 11:168–177
Vidal L, Pinsach J, Striedner G, Caminal G, Ferrer P (2008) Development of an antibiotic-free plasmid selection system based on glycine auxotrophy for recombinant protein overproduction in Escherichia coli. J Biotechnol 134:127–136
Fiedler M, Skerra A (2001) proBA complementation of an auxotrophic E. coli strain improves plasmid stability and expression yield during fermenter production of a recombinant antibody fragment. Gene 274:111–118
Kroll J, Klinter S, Steinbüchel A (2011) A novel plasmid addiction system for large-scale production of cyanophycin in Escherichia coli using mineral salts medium. Appl Microbiol Biotechnol 89:593–604
Gupta J, Pandey G, Mukherjee K (2001) Two-stage cultivation of recombinant Saccharomyces cerevisiae to enhance plasmid stability under non-selective conditions: experimental study and modeling. Enzym Microb Technol 28:89–99
Borsuk S, Mendum TA, Fagundes MQ, Michelon M, Cunha CW, McFadden J, Dellagostin OA (2007) Auxotrophic complementation as a selectable marker for stable expression of foreign antigens in Mycobacterium bovis BCG. Tuberc 87:474–480
Jensen PR, Hammer K (1998) The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Environ Microbiol 64:82–87
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. doi:10.1038/msb4100050
Spexard M, Beshay U, Risse JM, Miksch G, Flaschel E (2010) Screening for conditions of enhanced production of a recombinant beta-glucanase secreted into the medium by Escherichia coli. Biotechnol Lett 32:243–248
Beshay U, Miksch G, Flaschel E (2007) Improvement of a beta-glucanase activity test by taking into account the batch reactor balance of the test system. Bioprocess Biosyst Eng 30:251–259
Somers JM, Amzallag A, Middleton RB (1973) Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol 113:1268–1272
Sommer B, Friehs K, Flaschel E, Reck M, Stahl F, Scheper T (2009) Extracellular production and affinity purification of recombinant proteins with Escherichia coli using the versatility of the maltose binding protein. J Biotechnol 140:194–202
Notley L, Ferenci T (1996) Induction of RpoS-dependent functions in glucose-limited continuous culture: what level of nutrient limitation induces the stationary phase of Escherichia coli ? J Bacteriol 178:1465–1468
Kolter R, Siegele DA, Tormo A (1993) The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874
Teich A, Meyer S, Lin HY, Andersson L, Enfors S, Neubauer P (1999) Growth rate related concentration changes of the starvation response regulators σS and ppGpp in glucose-limited fed-batch and continuous cultures of Escherichia coli. Biotechnol Prog 15:123–129
Zgurskaya HI, Keyhan M, Matin A (1997) The σS level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol Microbiol 24:643–651
Utsumi R, Kusafuka S, Nakayama T, Tanaka K, Takayanagi Y, Takahashi H, Noda M, Kawamukai M (1993) Stationary phase-specific expression of the fic gene in Escherichia coli K-12 is controlled by the rpoS gene product (sigma 38). FEMS Microbiol Lett 113:273–278
Beshay U, El-Enshasy H, Ismail IM, Moawad H, Wojciechowska E, Abd-El-Ghany S (2003) β-Glucanase production from genetically modified recombinant Escherichia coli: effect of growth substrates and development of a culture medium in shake flasks and stirred tank bioreactor. Process Biochem 39:307–313
Beshay U, Miksch G, Friehs K, Flaschel E (2007) Improved β-glucanase production by a recombinant Escherichia coli strain using zinc-ion supplemented medium. Eng Life Sci 7:253–258
Popov M, Petrov S, Nacheva G, Ivanov I, Reichl U (2011) Effects of a recombinant gene expression on ColE1-like plasmid segregation in Escherichia coli. BMC Biotechnol. doi:10.1186/1472-6750-11-18
Glenting J, Madsen S, Vrang A, Fomsgaard A, Israelsen H (2002) A plasmid selection system in Lactococcus lactis and its use for gene expression in L. lactis and human kidney fibroblasts. Appl Environ Microbiol 68:5051–5056
Klumpp S (2011) Growth-rate dependence reveals design principles of plasmid copy number control. PLoS One. doi:10.1371/journal.pone.0020403
Meinander NQ, Hahn-Hägerdal B (1997) Fed-batch xylitol production with two recombinant Saccharomyces cerevisiae strains expressing XYL1 at different levels, using glucose as a cosubstrate: a comparison of production parameters and strain stability. Biotechnol Bioeng 54:391–399
Acknowledgments
The excellent guidance from Dr. Gerhard Miksch on discussions of the cloning strategy and for the gift of plasmid p582 is deeply acknowledged. Technical assistance from Eberhard Wünsch and Thomas Schäffer is gratefully acknowledged. R.S.V.S thanks the Deutscher Akademischer Austauschdienst (DAAD) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velur Selvamani, R.S., Friehs, K. & Flaschel, E. Extracellular recombinant protein production under continuous culture conditions with Escherichia coli using an alternative plasmid selection mechanism. Bioprocess Biosyst Eng 37, 401–413 (2014). https://doi.org/10.1007/s00449-013-1005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1005-4