Abstract
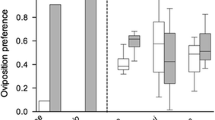
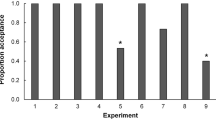
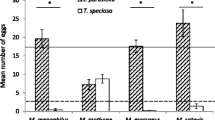
The preference-performance hypothesis posits that the host plant range of plant-feeding insects is ultimately limited by larval costs associated with feeding on multiple resources, and that female egg-laying preferences evolve in response to these costs. The trade-off of either using few host plant species and being a strong competitor on them due to effective utilization or using a wide host plant range but being a poor competitor is further predicted to result in host plant specialization. This follows under the hypothesis that both females and offspring are ultimately favoured by utilizing only the most suitable host(s). We develop an experimental approach to identify such trade-offs, i.e. larval costs associated with being a host generalist, and apply a suite of experiments to two sympatric and syntopic populations of the closely related butterflies Pieris napi and Pieris rapae. These butterflies show variation in their level of host specialization, which allowed comparisons between more and less specialized species and between families within species. Our results show that, first, the link between female host preference and offspring performance was not significantly stronger in the specialist compared to the generalist species. Second, the offspring of the host plant specialist did not outperform the offspring of the generalist on the former’s most preferred host plant species. Finally, the more generalized species, or families within species, did not show higher survival or consistently higher growth rates than the specialists on the less preferred plants. Thus, the preference and performance traits appear to evolve as largely separated units.



Similar content being viewed by others
References
Agrawal AA (2000a) Host range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500–508
Agrawal AA (2000b) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89:493–500
Barron AB (2001) The life and death of Hopkins’ host-selection principle. J Insect Behav 14:725–737
Berger D, Olofsson M, Gotthard K, Wiklund C, Friberg M (2012) Ecological constraints on female fitness in a phytophagous insect. Am Nat 180:464–480
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Bernays EA, Wcislo WT (1994) Sensory capabilities, information processing, and resource specialization. Q Rev Biol 69:187–204
Bolnick DI, Svanbäck R, Fordyce JA, Hang LH, Davis JM, Husley D, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Chew FS (1975) Coevolution of pierid butterflies and their cruciferous foodplants. I. The relative quality of available resources. Oecologia 20:117–127
Chew FS (1977) Coevolution of pierid butterflies and their cruciferous foodplants. II. The distribution of eggs on potential foodplants. Evolution 31:568–579
Chew FS (1981) Coexistence and local extinction in two pierid butterflies. Am Nat 118:655–672
Chew FS, Watt WB (2006) The green-veined white (Pieris napi L.), its Pierinae relatives, and the systematics dilemmas of diverging character sets (Lepidoptera, Pieridae). Biol J Linn Soc 88:413–435
Courtney SP (1983) Models of host plant location by butterflies: the effect of search images and searching efficiency. Oecologia 59:317–321
Courtney SP, Chew FS (1991) Plant apparency and evolutionary escape from insect herbivory. Am Nat 138:729–750
Dethier VG (1954) Evolution of feeding preferences in phytophagous insects. Evolution 8:33–54
Egan SP, Funk DJ (2006) Individual advantages to ecological specialization: insights on cognitive constraints from three conspecific taxa. Proc R Soc Lond Ser B Biol Sci 273:843–848
Emmet AM, Heath J (1989) The moths and butterflies of Great Britain and Ireland, vol. 7, part 1. Harley Books, Colchester, Essex, UK
Forsberg J (1987) Size discrimination among conspecific hostplants in two pierid butterflies; Pieris napi L. and Pontia dapliice L. Oecologia 72:52–57
Friberg M, Wiklund C (2009) Host plant preference and performance of the sibling species of butterflies Leptidea sinapis and Leptidea reali: a test of the trade-off hypothesis for food specialisation. Oecologia 159:127–137
Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C (2008) Habitat choice precedes host plant choice—niche separation in a species pair of a generalist and a specialist butterfly. Oikos 117:1337–1344
Friberg M, Aalberg Haugen IM, Dahlerus J, Gotthard K, Wiklund C (2011) Asymmetric life-history decision-making in butterfly larvae. Oecologia 165:301–310
Friberg M, Dahlerus J, Wiklund C (2012) Strategic larval decision-making in a bivoltine butterfly. Oecologia 169:623–635
Futuyma DJ (1979) Evolutionary biology, 1st edn. Sinauer, Sunderland
Futuyma D (1983) Selective factors in the evolution of host choice by phytophagous insects. In: Ahmad S (ed) Herbivorous insects: host seeking behavior and mechanisms. Academic Press, New York, pp 227–244
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference-performance relationships in phytophagous insects. Ecol Lett 13:383–393
Henriksen HJ, Kreutzer I (1982) Skandinaviens dagsommerfugle i naturen. Skandinavisk bokforlag, Odense
Hopkins AD (1917) A discussion of C. G. Hewitt’s paper on ‘insect behavior’. J Econ Entomol 10:92–93
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Janz N, Nylin S (1997) The role of female search behaviour in determining host plant range in plant feeding insects: a test of the information processing hypothesis. Proc R Soc Lond Ser B Biol Sci 264:701–707
Janz N, Söderlind L, Nylin S (2009) No effect of larval experience on adult host preferences in Polygonia c-album (Lepidoptera: Nymphalidae): on the persistence of Hopkins’ host selection principle. Ecol Entomol 34:50–57
Keeler MS, Chew FS (2008) Escaping an evolutionary trap: preference and performance of a native insect on an exotic invasive host. Oecologia 156:559–568
Levins R, MacArthur R (1969) An hypothesis to explain the incidence of monophagy. Ecology 5:910–911
Mayhew PJ (1997) Adaptive patterns of host plant selection by phytophagous insects. Oikos 79:417–428
Murphy SM (2004) Enemy-free space maintains swallowtail butterfly host shift. Proc Natl Soc USA 101:18048–18052
Nakajima M, Boggs CL, Bailey S, Reithel J, Paape T (2013) Fitness costs of butterfly oviposition on a lethal non-native plant in a mixed native and non-native plant community. Oecologia 172:823–832
Noriyuki S, Osawa N (2012) Intrinsic prey suitability in specialist and generalist Harmonia ladybirds: a test of the trade-off hypothesis for food specialization. Entomol Exp Appl 144:279–285
Ohsaki N, Sato Y (1994) Food plant choice of Pieris butterflies as a trade-off between parasitoid avoidance and quality of plants. Ecology 75:59–68
Pashalidou FG, Lucas-Barbosa D, Van Loon JJA, Dicke M, Fatouros NE (2013) Phenotypic plasticity of plant response to herbivore eggs: effects on resistance to caterpillars and plant development. Ecology 94:702–713
Rausher MD (1979) Larval habitat suitability and oviposition preference in three related butterflies. Ecology 60:503–511
Rausher MD (1984) Tradeoffs in performance on different hosts: evidence from within and between-site variation in the beetle Deloyala guttata. Evolution 38:582–595
Rausher MD (1988) Is coevolution dead? Ecology 69:898–901
Revelle W (2014) Psych: procedures for personality and psychological research, version 1.4.5. Northwestern University, Evanston, IL, http://CRAN.R-project.org/package
Sadeghi HS, Gilbert F (1999) Individual variation in oviposition preference, and its interaction with larval performance in an insect predator. Oecologia 118:405–411
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology (ed 2). Oxford University Press, Oxford
Scott JA (1986) The butterflies of North America. Stanford University Press, Stanford
Singer MC, Ng D, Thomas CD (1988) Heritability of oviposition preference and its relationship to offspring performance within a single insect population. Evolution 42:977–985
Slove J, Janz N (2011) The relationship between diet breadth and geographic range size in the butterfly subfamily Nymphalinae—a study of global scale. PLoS One 6:e16057
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Thomas CD, Ng D, Singer MC, Mallet JLB, Parmesan C, Billington HL (1987) Incorporation of a European weed into the diet of a North American herbivore. Evolution 41:892–901
StatSoft Inc. (2013) STATISTICA (data analysis software system), version 12. www.statsoft.com
Thöming G, Larsson MC, Hansson BS, Anderson P (2013) Comparison of plant preference hierarchies of male and female moths and the impact of larval rearing hosts. Ecology 94:1744–1752
Thompson JN (1988) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Wiklund C (1974) Oviposition preferences in Papilio machaon in relation to the host plants of the larvae. Entomol Exp Appl 17:189–198
Wiklund C (1975) The evolutionary relationship between adult oviposition preferences and larval host plant range in Papilio machaon. Oecologia 18:185–197
Wiklund C, Friberg M (2008) Enemy-free space and habitat-specific host specialization in a butterfly. Oecologia 157:287–294
Wiklund C, Friberg M (2009) The evolutionary ecology of generalization: among-year variation in host plant use and offspring survival in a butterfly. Ecology 90:3406–3417
Wiklund C, Wickman P-O, Nylin S (1992) A sex difference in the propensity to enter direct/diapause development—a result of selection for protandry? Evolution 46:519–528
Wiklund C, Karlsson B, Leimar O (2001) Sexual conflict and cooperation in butterfly reproduction: a comparative study of polyandry and female fitness. Proc R Soc Lond Ser B Biol Sci 268:1661–1667
Acknowledgments
We thank Åsa Sandström for valuable help in butterfly rearing, Toomas Tammaru, Lee Dyer and two anonymous reviewers for useful comments on earlier versions of this manuscript. This study was funded by the Royal Swedish Academy of Agriculture and Forestry, the Stina Werner Foundation and H. M. the King’s 50th Birthday Fund to M. F. and by the Swedish Research Council to C. W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Klaus Fischer.
Rights and permissions
About this article
Cite this article
Friberg, M., Posledovich, D. & Wiklund, C. Decoupling of female host plant preference and offspring performance in relative specialist and generalist butterflies. Oecologia 178, 1181–1192 (2015). https://doi.org/10.1007/s00442-015-3286-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3286-6