Abstract
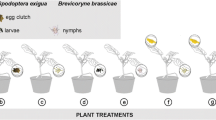
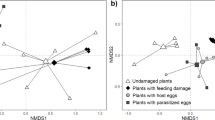
Animals use information from their environment while foraging for food or prey. When parasitic wasps forage for hosts, they use plant volatiles induced by herbivore activities such as feeding and oviposition. Little information is available on how wasps exploit specific plant volatiles over time, and which compounds indicate changes in host quality. In experiments investigating the role of herbivore-induced plant volatiles in wasp foraging, induction of plant response is usually achieved by placing larvae on clean plants instead of allowing the natural sequence of events: to let eggs deposited by the herbivore develop into larvae. We compared the attraction of the parasitoid Cotesia glomerata to volatiles emitted by black mustard (Brassica nigra) plants induced by eggs and successive larval stages of the Large Cabbage White butterfly (Pieris brassicae) to the attraction of this parasitoid to black mustard plant volatiles induced only by larval feeding in a wind tunnel setup. We show that wasps are attracted to plants infested with eggs just before and shortly after larval hatching. However, wasp preference changed at later time points towards plants induced only by larval feeding. These temporal changes in parasitoid attraction matched with changes in the chemical compositions of the blends of plant volatiles. Previous studies have shown that host quality/suitability decreases with caterpillar age and that P. brassicae oviposition induces plant defences that negatively affect subsequently feeding caterpillars. We investigated parasitoid performance in hosts of different ages. Wasp performance was positively correlated with preference. Moreover, parasitism success decreased with time and host stage. In conclusion, the behaviour of Cotesia glomerata is fine-tuned to exploit volatiles induced by eggs and early host stages that benefit parasitoid fitness.



Similar content being viewed by others
References
Amo L, Jansen JJ, van Dam NM, Dicke M, Visser ME (2013) Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol Lett 16:1348–1355
Brodeur J, Geervliet JBF, Vet LEM (1998) Effects of Pieris host species on life history parameters in a solitary specialist and gregarious generalist parasitoid (Cotesia species). Entomol Exp Appl 86:145–152
Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–317
Clavijo McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
De Boer J, Posthumus M, Dicke M (2004) Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 30:2215–2230
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–579
de Rijk M, Dicke M, Poelman EH (2013) Foraging behaviour by parasitoids in multiherbivore communities. Anim Behav 85:1517–1528
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Fatouros NE, Bukovinszkine’Kiss G, Kalkers LA, Gamborena RS, Dicke M, Hilker M (2005) Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomologia Experimentalis et Applicata 115:207–215
Fatouros NE et al (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607
Fatouros NE, Pineda A, Huigens ME, Broekgaarden C, Shimwela MM, Figueroa IA, Verbaarschot P, Bukovinszky T (2014) Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proc R Soc B Biol Sci 281:20141254. doi:10.1098/rspb.2014.1254
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824
Gols R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA (2008) Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J Chem Ecol 34:132–143
Gouinguené S, Alborn H, Turlings TC (2003) Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J Chem Ecol 29:145–162
Harvey JA (2005) Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl 117:1–13
Harvey JA, Jervis MA, Gols R, Jiang N, Vet LEM (1999) Development of the parasitoid, Cotesia rubecula (Hymenoptera: Braconidae) in Pieris rapae and Pieris brassicae (Lepidoptera: Pieridae): evidence for host regulation. J Insect Physiol 45:173–182
Harvey JA, Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31:287–302
Hern A, Dorn S (2001) Induced emissions of apple fruit volatiles by the codling moth: changing patterns with different time periods after infestation and different larval instars. Phytochemistry 57:409–416
Hilker M, Meiners T (2010) How do plants “notice” attack by herbivorous arthropods? Biol Rev Camb Philos Soc 85:267–280
Hilker M, Meiners T (2011) Plants and insect eggs: how do they affect each other? Phytochemistry 72:1612–1623
Jervis MA, Ellers J, Harvey JA (2008) Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu Rev Entomol 53:361–385
Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Funct Ecol 25:348–357
Kim J, Felton GW (2013) Priming of antiherbivore defensive responses in plants. Insect Sci 20:273–285
Kopke D, Schroder R, Fischer HM, Gershenzon J, Hilker M, Schmidt A (2008) Does egg deposition by herbivorous pine sawflies affect transcription of sesquiterpene synthases in pine? Planta 228:427–438
Kopke D, Beyaert I, Gershenzon J, Hilker M, Schmidt A (2010) Species-specific responses of pine sesquiterpene synthases to sawfly oviposition. Phytochemistry 71:909–917
Loughrin JH, Manukian A, Heath RR, Turlings TC, Tumlinson JH (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA 91:11836–11840
Lucas-Barbosa D, Poelman E, Aartsma Y, Snoeren TL, van Loon JA, Dicke M (2014) Caught between parasitoids and predators—survival of a specialist herbivore on leaves and flowers of mustard plants. J Chem Ecol 40:621–631
Mathur V et al (2013) An ecogenomic analysis of herbivore-induced plant volatiles in Brassica juncea. Mol Ecol 22:6179–6196
Mattiacci L, Dicke M (1995a) Host-age discrimination during host location by Cotesia glomerata, a larval parasitoid of Pieris brassicae. Entomol Exp Appl 76:37–48
Mattiacci L, Dicke M (1995b) The parasitoid Cotesia glomerata (Hymenoptera: Braconidae) discriminates between first and fifth larval instars of its host Pieris brassicae, on the basis of contact cues from frass, silk, and herbivore-damaged leaf tissue. J Insect Behav 8:485–498
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667
Pashalidou FG, Lucas-Barbosa D, van Loon JJA, Dicke M, Fatouros NE (2013) Phenotypic plasticity of plant response to herbivore eggs: effects on resistance to caterpillars and plant development. Ecology 94:702–713
Peñaflor MFGV et al (2011) Oviposition by a moth suppresses constitutive and herbivore-induced plant volatiles in maize. Planta 234:207–215
Ping L-Y, Y-B Shen, Y-J Jin (2001) Volatiles released in succession from artificially damaged ashleaf maple leaves. Funct Plant Biol 28:513–517
Pinto-Zevallos DM, Hellén H, Hakola H, van Nouhuys S, Holopainen JK (2013) Induced defenses of Veronica spicata: variability in herbivore-induced volatile organic compounds. Phyt Lett 6:653–656
Poelman EH, van Loon JJ, Dicke M (2008) Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci 13:534–541
Ponzio CGR, Berhane TW, Dicke M (2014) Caterpillar-induced plant volatiles remain a reliable signal for foraging wasps during dual attack with a plant pathogen or non-host insect herbivore. Plant Cell Environ 37:1924–1938
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Reymond P (2013) Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 238:247–258
Scascighini N, Mattiacci L, D’Alessandro M, Hern A, Sybille Rott A, Dorn S (2005) New insights in analysing parasitoid attracting synomones: early volatile emission and use of stir bar sorptive extraction. Chemoecology 15:97–104
Smallegange RC, van Loon JJ, Blatt SE, Harvey JA, Agerbirk N, Dicke M (2007) Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J Chem Ecol 33:1831–1844
Snoeren TAL, De Jong PW, Dicke M (2007) Ecogenomic approach to the role of herbivore-induced plant volatiles in community ecology. J Ecol 95:17–26
Stam JM et al (2014) Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol 65:689–713
Takabayashi J, Takahashi S, Dicke M, Posthumus MA (1995) Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol 21:273–287
Tamiru A, Bruce TJA, Woodcock CM, Caulfield JC, Midega CAO, Ogol CKPO, Mayon P, Birkett MA, Pickett JA, Khan ZR (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14:1075–1083
Tentelier C, Fauvergue X (2007) Herbivore-induced plant volatiles as cues for habitat assessment by a foraging parasitoid. J Anim Ecol 76:1–8
Turlings TC, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207:146–152
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst 58:109–130
Acknowledgments
The authors thank Léon Westerd, Joop Woelke and André Gidding for rearing insects, Unifarm of Wageningen University for providing plants, and the Netherlands Organization for Scientific Research (NWO/ALW VENI grant 863.09.002 to N.E. Fatouros and NWO/ALW grant 820.01.022 to M. Dicke) for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Moshe Inbar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pashalidou, F.G., Gols, R., Berkhout, B.W. et al. To be in time: egg deposition enhances plant-mediated detection of young caterpillars by parasitoids. Oecologia 177, 477–486 (2015). https://doi.org/10.1007/s00442-014-3098-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3098-0