Abstract
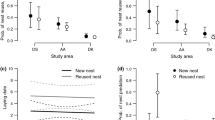
Many organisms of temperate latitudes exhibit declines in reproductive success as the breeding season advances. Experiments can delay the onset of reproduction for early breeders to investigate the consequences of late nesting, but it is rarely possible to observe a distinct second round of nesting in species that normally nest only once. The colonial cliff swallow (Petrochelidon pyrrhonota) is a migratory songbird that has a relatively short breeding season in the western Great Plains, USA, with birds rarely nesting late in the summer. Previous work suggested that ectoparasitism is a primary reason why reproductive success in this species declines over the summer. At colony sites where nests were fumigated to remove ectoparasitic swallow bugs (Oeciacus vicarius), cliff swallows frequently undertook a distinct round of late nesting after previously fledging young that year. Mark-recapture revealed that late-nesting pairs at these colonies produced fewer offspring that survived to the next breeding season, and that survival of late-nesting adults was lower during the next year, relative to pairs nesting earlier in the season. These reproductive costs applied in the absence of ectoparasites and likely reflect other environmental costs of late nesting such as seasonal declines in food availability or a delayed start of fall migration. Despite the costs, the estimated fitness for perennial early-and-late nesters in the absence of ectoparasites was equivalent to that of birds that nested only early in the season. The collective disadvantages of late nesting likely constrain most cliff swallows to raising a single brood in the middle latitudes of North America.

Similar content being viewed by others
References
Brinkhof MWG, Cave AJ, Perdeck AC (1997) The seasonal decline in the first-year survival of juvenile coots: an experimental approach. J Anim Ecol 66:73–82
Brown CR (1978) Double-broodedness in purple martins in Texas. Wilson Bull 90:239–247, 657
Brown CR, Brown MB (1986) Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67:1206–1218
Brown CR, Brown MB (1995) Cliff swallow (Hirundo pyrrhonota). In: Poole A, Gill F (eds) The birds of North America. Academy of Natural Sciences, Philadelphia, PA, and American Ornithologists’ Union, Washington DC
Brown CR, Brown MB (1996) Coloniality in the cliff swallow: the effect of group size on social behavior. University of Chicago Press, Chicago
Brown CR, Brown MB (1999) Fitness components associated with laying date in the cliff swallow. Condor 101:230–245
Brown CR, Brown MB (2004a) Empirical measurement of parasite transmission between groups in a colonial bird. Ecology 85:1619–1626
Brown CR, Brown MB (2004b) Group size and ectoparasitism affect daily survival probability in a colonial bird. Behav Ecol Sociobiol 56:498–511
Brown CR, Brown MB, Moore A, Komar N (2007) Bird movement predicts Buggy Creek virus infection in insect vectors. Vector-Borne Zoonotic Dis 7:304–314
Brown CR, Brown MB, Roche EA (2013) Spatial and temporal unpredictability of colony size in cliff swallows across 30 years. Ecol Monogr 83:511–530
Brownie C, Anderson DR, Burnham KP, Robson DS (1985) Statistical inference from band recovery data―a handbook, 2nd edn. Research publication 156. United States Department of the Interior, Washington DC
Chapman BR, George JE (1991) The effects of ectoparasites on cliff swallow growth and survival. In: Loye JE, Zuk M (eds) Bird–parasite interactions: ecology. evolution and behavior. Oxford University Press, Oxford, pp 69–92
Clayton DH, Moore J (1997) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford
Dawson A, Hinsley SA, Ferns PN, Bonser RHC, Eccleston L (2000) Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proc R Soc Lond B 267:2093–2098
de Lope F, González G, Pérez JJ, Møller AP (1993) Increased detrimental effects of ectoparasites on their bird hosts during adverse environmental conditions. Oecologia 95:234–240
Emlen ST, Demong NJ (1975) Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis. Science 188:1029–1031
Geupel GR, DeSante DF (1990) Incidence and determinants of double brooding in wrentits. Condor 92:67–75
Hansson B, Bensch S, Hasselquist D (2000) The quality and the timing hypotheses evaluated using data on great reed warblers. Oikos 90:575–581
Hochachka W (1990) Seasonal decline in reproductive performance of song sparrows. Ecology 71:1279–1288
Husby A, Kruuk LEB, Visser ME (2009) Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proc R Soc B 276:1845–1854
Imlay TJ, Crowley JF, Argue AM, Steiner JC, Norris DR, Stutchbury BJM (2010) Survival, dispersal and early migration movements of captive-bred juvenile eastern loggerhead shrikes (Lanius ludovicianus migrans). Biol Conserv 143:2578–2582
Jacobs AC, Reader LL, Fair JM (2013) What determines the rates of double brooding in the western bluebird? Condor 115:386–393
Klomp H (1970) The determinants of clutch size in birds: a review. Ardea 58:1–125
Lapolla A, Buckley LJ (2005) Hatch date distributions of young-of-year haddock Melanogrammus aeglefinus in the Gulf of Maine/Georges Bank region: implications for recruitment. Mar Ecol Prog Ser 290:239–249
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Loye JE, Zuk M (eds) (1991) Bird-parasite interactions: ecology, evolution and behaviour. Oxford University Press, Oxford
Magrath MJL, Santema P, Bouwman KM, Brinkhuizen DM, Griffith SC, Langemore NE (2009) Seasonal decline in reproductive performance varies with colony size in the fairy martin, Petrochelidon ariel. Behav Ecol Sociobiol 63:661–672
Mahony NA, Vander Haegen WM, Walker BL, Krannitz PG (2001) Male incubation and multiple brooding in sagebrush Brewer’s sparrows. Wilson Bull 113:441–444
Møller AP (1990) Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71:2345–2357
Møller AP, Allander K, Dufva R (1990) Fitness effects of parasites on passerine birds: a review. In: Blondel J, Gosler A, Lebreton JD, McCleery R (eds) Population biology of passerine birds. Springer, Berlin, pp 269–280
Møller AP, Merino S, Brown CR, Robertson RJ (2001) Immune defense and host sociality: a comparative study of swallows and martins. Am Nat 158:136–145
Monroe AP, Hallinger KK, Brasso RL, Cristol DA (2008) Occurrence and implications of double brooding in a southern population of tree swallows. Condor 110:382–386
Nagy LR, Holmes RT (2005) To double-brood or not? Individual variation in the reproductive effort in black-throated blue warblers (Dendroica caerulescens). Auk 122:902–914
Nilsson JA, Svensson E (1996) The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc R Soc Lond B 263:711–714
Nolan V Jr (1978) The ecology and behavior of the prairie warbler Dendroica discolor. Ornithol Monogr 26. American Ornithologists’ Union, Washington DC
Perrins CM (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Powell LA (2007) Approximating variance of demographic parameters using the delta method: a reference for avian biologists. Condor 109:949–954
Price T, Kirkpatrick M, Arnold SJ (1988) Directional selection and the evolution of breeding date in birds. Science 240:798–799
Ramstack JM, Murphy MT, Palmer MR (1998) Comparative reproductive biology of three species of swallows in a common environment. Wilson Bull 110:233–243
Richner H, Heeb P (1995) Are clutch and brood size patterns in birds shaped by ectoparasites? Oikos 73:435–441
Roche EA, Brown CR, Brown MB, Lear KM (2013) Recapture heterogeneity in cliff swallows: increased exposure to mist nets leads to net avoidance. PLoS One 8:e58092
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and the seasonal decline of avian clutch size. Am Nat 143:698–722
Sanz JJ (1999) Seasonal variation in reproductive success and post-nuptial moult of blue tits in southern Europe: an experimental study. Oecologia 121:377–382
Svensson E (1997) Natural selection on avian breeding time: causality, fecundity-dependent, and fecundity-independent selection. Evolution 51:1276–1283
Underhill LG, Prys-Jones RP, Dowsett RJ, Herroelen P, Johnsonn DN, Lawn MR, Norman SC, Pearson DJ, Tree AJ (1992) The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis 134:286–297
Vega Rivera JH, Rappole JH, McShea WJ, Haas CA (1998) Wood thrush postfledging movements and habitat use in northern Virginia. Condor 100:69–78
Verboven N, Verhulst S (1996) Seasonal variation in the incidence of double broods: the date hypothesis fits better than the quality hypothesis. J Anim Ecol 65:264–273
Verhulst S, Nilsson JA (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc B 363:399–410
Verhulst S, van Balen JH, Tinbergen JM (1995) Seasonal decline in reproductive success of the great tit: variation in time or quality? Ecology 76:2392–2403
Wardrop SL, Ydenberg RC (2003) Date and parental quality effects in the seasonal decline in reproductive performance of the tree swallow Tachycineta bicolor: interpreting results in light of potential experimental bias. Ibis 145:439–447
Weaver HB, Brown CR (2004) Brood parasitism and egg transfer in cave swallows (Petrochelidon fulva) and cliff swallows (P. pyrrhonota) in south Texas. Auk 121:1122–1129
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–S139
Acknowledgments
We thank Frank Axell, Alex Brazeal, Ananda Ellis, Emily Geary, Beate Hall, Bryce Hatfield, Jordan Herman, Allison Johnson, Kristen Lear, Nathan Miller, Catherine Page, Paul Smelcer, and Jessica Tipple for assistance with mark-recapture. Amy Moore managed the mark-recapture database. The Union Pacific Railroad provided access to land. The School of Biological Sciences at the University of Nebraska-Lincoln allowed us to use the Cedar Point Biological Station. We thank the National Science Foundation (DEB-0514824, DEB-1019423), the National Institutes of Health (AI057569), and the University of Tulsa’s Graduate School for financial support. This work was approved by the University of Tulsa’s Institutional Animal Care and Use Committee under protocol TU-0020 and was conducted in accordance with the laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver P. Love.
Rights and permissions
About this article
Cite this article
Brown, C.R., Roche, E.A. & O’Brien, V.A. Costs and benefits of late nesting in cliff swallows. Oecologia 177, 413–421 (2015). https://doi.org/10.1007/s00442-014-3095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3095-3