Abstract
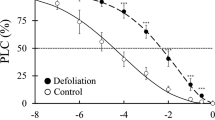
Plant fitness is often defined by the combined effects of herbivory and competition, and plants must strike a delicate balance between their ability to capture limiting resources and defend against herbivore attack. Many plants use indirect defenses, such as volatile compounds and extrafloral nectaries (EFN), to attract canopy arthropods that are natural enemies of herbivorous organisms. While recent evidence suggests that upon perception of low red to far-red (R:FR) ratios, which signal the proximity of competitors, plants down-regulate resource allocation to direct chemical defenses, it is unknown if a similar phytochrome-mediated response occurs for indirect defenses. We evaluated the interactive effects of R:FR ratio and simulated herbivory on nectar production by EFNs of passionfruit (Passiflora edulis f. flavicarpa). The activity of petiolar EFNs dramatically increased in response to simulated herbivory and hormonal treatment with methyl jasmonate (MeJA). Low R:FR ratios, which induced a classic “shade-avoidance” repertoire of increased stem elongation in P. edulis, strongly suppressed the EFN response triggered by simulated herbivory or MeJA application. Strikingly, the EFN response to wounding and light quality was localized to the branches that received the treatments. In vines like P. edulis, a local response would allow the plants to precisely adjust their light harvesting and defense phenotypes to the local conditions encountered by individual branches when foraging for resources in patchy canopies. Consistent with the emerging paradigm that phytochrome regulation of jasmonate signaling is a central modulator of adaptive phenotypic plasticity, our results demonstrate that light quality is a strong regulator of indirect defenses.









Similar content being viewed by others
References
Agrawal A, Kearney E, Hastings A, Ramsey T (2012) Attenuation of the jasmonate burst, plant defensive traits, and resistance to specialist monarch caterpillars on shaded common milkweed (Asclepias syriaca). J Chem Ecol 38:893–901
Apple JL, Feener DH Jr (2001) Ant visitation of extra floral nectaries of Passiflora: the effects of nectary attributes and ant behavior on patterns in facultative ant-plant mutualisms. Oecologia 127:409–416
Ballaré CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4:97–102
Ballaré CL (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32:713–725
Ballaré CL (2011) Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci 16:249–257
Ballaré CL, Scopel AL, Roush ML, Radosevich SR (1995) How plants find light in patchy canopies. A comparison between wild-type and phytochrome-B-deficient mutant plants of cucumber. Funct Ecol 9:859–868
Ballaré CL, Mazza CA, Austin AT, Pierik R (2012) Canopy light and plant health. Plant Physiol 160:145–155
Bentley BL (1977) Extra-floral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Syst 8:407–427
Bernays EA, Cornelius ML (1989) Generalist caterpillar prey are more palatable than specialists for the generalist predator Iridomyrmex humilis. Oecologia 79:427–430
Bixenmann RJ, Coley PD, Kursar TA (2011) Is extra floral nectar production induced by herbivores or ants in a tropical facultative ant-plant mutualism? Oecologia 165:417–425
Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ (2012) Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol 160:1110–1119
Casal JJ (2012) Shade Avoidance. In: The Arabidopsis Book. American Society of Plant Biologists, Rockville, p e0157
Cerrudo I et al (2012) Low red/far-red ratios reduce arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol 158:2042–2052
Chamberlain SA, Holland NJ (2009) Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology 90:2384–2392
Cornelius ML, Bernays EA (1995) The effect of plant chemistry on the acceptability of caterpillar prey to the Argentine ant Iridomyrmex humilils (Hymenoptera: Formicidae). J Insect Behav 8:579–593
De Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell Environ 32:704–712
De Wit M et al (2013) Perception of low red: far-red ratio compromises both salicylic acid- and Jasmonic acid- dependent pathogen defences in Arabidopsis. Plant J 75:90–103
Deginani NB (2001) Las especies argentinas del género Passiflora (Passifloraceae). Darwiniana 39:43–129
DeLucia EH, Nabity PD, Zavala JA, Berenbaum MR (2012) Climate change: resetting plant-insect interactions. Plant Physiol 160:1677–1685
Di Giusto B, Anstett MC, Dounias E, McKey DB (2001) Variation in the effectiveness of biotic defence: the case of an opportunistic ant-plant protection mutualism. Oecologia 129:367–375
Escalante-Pérez M et al (2012) Poplar extra floral nectaries: two types, two strategies of indirect defenses against herbivores. Plant Physiol 159:1176–1191
Harper JL (1977) Population biology of plants. Academic, London
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Heil M (2010) Plastic defence expression in plants. Evol Ecol 24:555–569
Heil M (2011) Nectar: generation, regulation and ecological functions. Trends Plant Sci 16:191–200
Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE (2001) Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA 98:1083–1088
Held M, Baldwin IT (2005) Soil degradation slows growth and inhibits jasmonate-induced resistance in Artemisia vulgaris. Ecol Appl 15:1689–1700
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL (2003) Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132:1755–1767
Izaguirre MM, Mazza CA, Biondini M, Baldwin IT, Ballaré CL (2006) Remote sensing of future competitors: impacts on plant defenses. Proc Natl Acad Sci USA 103:7170–7174
Karban R, Baldwin I (1997) Induced responses to herbivory. Chicago University Press, Chicago
Kazan K, Manners JM (2011) The interplay between light and jasmonate signalling during defence and development. J Exp Bot 62:4087–4100
Kegge W, Pierik R (2010) Biogenic volatile organic compounds and plant competition. Trends Plant Sci 15:126–132
Keller MM, Jaillais Y, Pedmale UV, Moreno JE, Chory J, Ballaré CL (2011) Crypto chrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially-independent hormonal cascades. Plant J 67:195–207
Kigathi RN, Weisser WW, Veit D, Gershenzon J, Unsicker SB (2013) Plants suppress their emission of volatiles when growing with conspecifics. J Chem Ecol 39:537–545
Koptur S (1974) Facultative mutualism between weedy vetches bearing extra floral nectaries and weedy ants in California. Am J Bot 66:1016–1020
McGuire R, Agrawal AA (2005) Trade-offs between the shade-avoidance response and plant resistance to herbivores? Tests with mutant Cucumis sativus. Funct Ecol 19:1025–1031
McLain DK (1983) Ants, extra floral nectaries and herbivory on the Passion vine, Passiflora incarnata. Am Midl Nat 110:433–439
Moreno JE, Tao Y, Chory J, Ballaré CL (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106:4935–4940
Novoplansky A (2009) Picking battles wisely: plant behaviour under competition. Plant, Cell Environ 32:726–741
Pierik R, Mommer L, Voesenek LACJ (2013) Molecular mechanisms of plant competition: neighbour detection and response strategies. Funct Ecol. doi:10.1111/1365-2435.12010
Radhika V, Kost C, Mithöfer A, Boland W (2010) Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proc Natl Acad Sci USA 107:17228–17233
Ray TS (1992) Foraging behaviour in tropical herbaceous climbers (Araceae). J Ecol 80:189–203
Roberts MR, Paul ND (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170:677–699
Ruberti I, Sessa G, Ciolfi A, Possenti M, Carabelli M, Morelli G (2012) Plant adaptation to dynamically changing environment: the shade avoidance response. Biotechnol Adv 30:1047–1058
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology, 2nd edn. Oxford University Press, New York
Smiley J (1986) Ant constancy at Passiflora extra floral nectaries: effects on caterpillar survival. Ecology 67:516–521
Stephenson AG (1982) The role of the extra floral nectaries of Catalpa speciosa in limiting herbivory and increasing fruit production. Ecology 63:663–669
Tao Y et al (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176
Way MJ, Paiva MR, Cammell ME (1999) Natural biological control of the pine processionary moth Thaumetopoea pityocampa (Den. & Schiff.) by the Argentine ant Linepithema humile (Mayr) in Portugal. Agric For Entomol 1:27–31
Xu FF, Chen J (2010) Competition hierarchy and plant defense in a guild of ants on tropical Passiflora. Insectes Soc 57:343–349
Acknowledgments
This work was financially supported by CONICET, ANPCyT and UBACyT. We thank Amy Austin for thoughtful comments on the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell K Monson.
Rights and permissions
About this article
Cite this article
Izaguirre, M.M., Mazza, C.A., Astigueta, M.S. et al. No time for candy: passionfruit (Passiflora edulis) plants down-regulate damage-induced extra floral nectar production in response to light signals of competition. Oecologia 173, 213–221 (2013). https://doi.org/10.1007/s00442-013-2721-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2721-9