Abstract
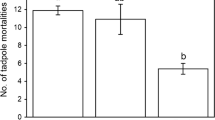
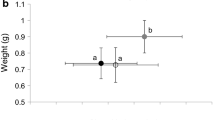
Laboratory experiments are widely used to study how populations in nature might respond to various biological interactions, but the relevance of experiments in artificial venues is not known. We compiled mortality and growth data from 424 anuran populations carried out under laboratory, mesocosm, field enclosure, and field settings to determine if major differences exist amongst experimental venues and how this might influence experimental responses of tadpoles amongst venues. Our results show that there are fundamental differences in survival amongst venues, with the highest mortality occurring in field populations and the lowest in laboratory populations. Separation of mesocosm and field enclosure data based on the possibility of predatory interactions indicates that predation is an important factor leading to increased mortality in natural populations. Comparisons of size distributions across venues (although size data were limited for field populations) suggest that variation in tadpole size is low in natural populations compared to populations in artificial venues. We infer from this that mortality has a homogenizing effect on size in nature, resulting in natural populations that are not a random sample of hatched individuals. This finding suggests that populations reared under controlled laboratory conditions in the absence of predation (and other selective pressures) may not be representative of natural populations.






Similar content being viewed by others
References
Bardsley L, Beebee TJC (1998) Interspecific competition between larvae is not an important structuring force in mixed communities of Rana and Bufo on an English sand-dune system. Ecography 21:449–456
Berven KA (1990) Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71:1599–1608
Berven KA, Chadra BG (1988) The relationship among egg size, density and food level on larval development in the wood frog (Rana sylvatica). Oecologia 75:67–72
Calef GW (1973) Natural mortality of tadpoles in a population of Rana aurora. Ecology 54:741–758
Calisi RM, Bentley GE (2009) Lab and field experiments: are they the same animal? Horm Behav 56:1–10
Callery EM (2006) There’s more than one frog in the pond: a survey of the amphibia and their contributions to developmental biology. Semin Cell Dev Biol 17:80–92
Carpenter SR (1989) Replication and treatment strength in whole-lake experiments. Ecology 70:453–463
Carpenter SR (1996) Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 77:677–680
Carpenter SR (1999) Microcosm experiments have limited relevance for community and ecosystem ecology: reply. Ecology 80:1085–1088
Cecil SG, Just JJ (1979) Survival rate, population density and development of a naturally occurring Anuran larvae (Rana catesbeiana). Copeia 1979:447–453
Chalcraft DR, Binckley CA, Resetarits WJ (2005) Experimental venue and estimation of interaction strength: comment. Ecology 86:1061–1067
Gascon C, Travis J (1992) Does the spatial scale of experimentation matter? a test with tadpoles and dragonflies. Ecology 73:2237–2243
Gause GF (1934) The struggle for existence. Williams & Wilkins, Baltimore
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analysis. Ecology 80(4):1142–1149
Haag D, Matschonat G (2001) Limitations of controlled experimental systems as models for natural systems: a conceptual assessment of experimental practices in biogeochemistry and soil science. Sci Total Environ 277:199–216
Hairston N (1989) Hard choices in ecological experimentation. Herpetologica 45:119–122
Herreid CF, Kenney S (1966) Survival of Alaskan woodfrog (Rana sylvatica) larvae. Ecology 47:1039–1041
Huffaker CB (1958) Experimental studies on predation: dispersion factors and predator–prey oscillations. Hilgardia 27:343–383
Jaeger RG, Walls SC (1989) Review: on salamander guilds and ecological methodology. Herpetologica 45:111–119
Keddy PA (2001) Competition, 2nd edn. Kluwer, Dordrecht
Kissner KJ, Weatherhead PJ (2005) Phenotypic effects on survival of neonatal northern watersnakes Nerodia sipendon. J Anim Ecol 74:259–265
Lajeunesse MJ (2009) Meta-analysis and the comparative phylogenetic method. Am Nat 174(3):369–381
Loman J (2004) Density regulation in tadpoles of Rana temporaria: a full pond field experiment. Ecology 85:1611–1618
Morin PJ (1989) New directions in amphibian community ecology. Herpetologica 45:124–128
Morin PJ (1999) Community ecology. Blackwell, Malden
OECD (2008) OECD guidelines for the testing of chemicals—the amphibian metamorphosis assay. In: Series on testing and assessment. Environmental Health and Safety Publications, OECD, Paris
Osenberg CW, Sarnelle O, Cooper SD, Holt RD (1999) Resolving ecological questions through meta-analysis: goals, metrics, and models. Ecology 80(4):1105–1117
Peacor SD, Pfister CA (2006) Experimental and model analyses of the effects of competition on individual size variation in wood frog (Rana sylvatica) tadpoles. J Anim Ecol 75:990–999
Perez KO, Munch SB (2010) Extreme selection on size in the early lives of fish. Evolution 68:2450–2457
Semlitsch RD (1990) Effects of body size, sibship, and tail injury on the susceptibility of tadpoles to dragonfly predation. Can J Zool 68:1027–1030
Semlitsch RD, Gibbons JW (1988) Fish predation in size-structured populations of treefrog tadpoles. Oecologia 75:321–326
Skelly DK (2002) Experimental venue and estimation of interaction strength. Ecology 83:2097–2101
Skelly DK (2005) Experimental venue and estimation of interaction strength: reply. Ecology 86:1068–1071
Thurnheer S, Reyer H-U (2001) Spatial distribution and survival rate of waterfrog tadpoles in relation to biotic and abiotic factors: a field experiment. Amphibia-Reptilia 22:21–32
Turner FB (1960) Population structure and dynamics of the western spotted frog, Rana p. pretiosa Baird & Girard, in Yellowstone Park Wyoming. Ecol Monogr 30:251–278
Wilbur HM (1976) Density-dependent aspects of metamorphosis in Ambystoma and Rana sylvatica. Ecology 57:1289–1296
Wilhelm FM, Schindler DW, McNaught AS (2000) The influence of experimental scale on estimating the predation rate of Gammarus lacustris (crustacea: amphipoda) on daphnia in an alpine lake. J Plankton Res 22:1719–1734
Acknowledgments
We would like to acknowledge and thank the authors of the original studies included in our analysis for making this review possible. We also thank two anonymous reviewers for excellent feedback that improved the manuscript. This work was funded through the National Science and Engineering Research Council (NSERC) Discovery grant to Jeff Houlahan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Melvin, S.D., Houlahan, J.E. Tadpole mortality varies across experimental venues: do laboratory populations predict responses in nature?. Oecologia 169, 861–868 (2012). https://doi.org/10.1007/s00442-012-2260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2260-9