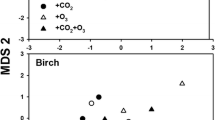
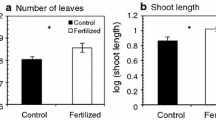
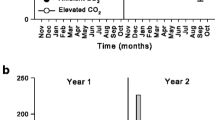
Abstract
This study examined the independent and interactive effects of elevated carbon dioxide (CO2) and ozone (O3) on the foliar quality of two deciduous trees species and the performance of two outbreak herbivore species. Trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera) were grown at the Aspen FACE research site in northern Wisconsin, USA, under four combinations of ambient and elevated CO2 and O3. We measured the effects of elevated CO2 and O3 on aspen and birch phytochemistry and on gypsy moth (Lymantria dispar) and forest tent caterpillar (Malacosoma disstria) performance. Elevated CO2 nominally affected foliar quality for both tree species. Elevated O3 negatively affected aspen foliar quality, but only marginally influenced birch foliar quality. Elevated CO2 slightly improved herbivore performance, while elevated O3 decreased herbivore performance, and both responses were stronger on aspen than birch. Interestingly, elevated CO2 largely offset decreased herbivore performance under elevated O3. Nitrogen, lignin, and C:N were identified as having strong influences on herbivore performance when larvae were fed aspen, but no significant relationships were observed for insects fed birch. Our results support the notion that herbivore performance can be affected by atmospheric change through altered foliar quality, but how herbivores will respond will depend on interactions among CO2, O3, and tree species. An emergent finding from this study is that tree age and longevity of exposure to pollutants may influence the effects of elevated CO2 and O3 on plant–herbivore interactions, highlighting the need to continue long-term atmospheric change research.

Similar content being viewed by others
References
Agrell J, Kopper BJ, McDonald EP, Lindroth RL (2005) CO2 and O3 effects on host plant preferences of the forest tent caterpillar (Malacosoma disstria). Glob Change Biol 11:588–599
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Barbehenn RV, Jaros A, Lee G, Mozola C, Weihr Q, Salminen J-P (2009) Tree resistance to Lymantria dispar caterpillars: importance and limitations of foliar tannin composition. Oecologia 159:777–788
Betz GA, Gerstner E, Stich S, Winkler B, Welzi G, Kremmer E, Langebartles C, Heller W, Sandermann H, Ernst D (2009) Ozone affects shikimate pathway genes and secondary metabolites in saplings of European birch (Fagus sylvatica L.) grown under greenhouse conditions. Trees Struct Funct 23:539–553
Bezemer TM, Jones TH (1998) Plant–insect herbivore interactions in elevated CO2: quantitative analysis and guild effects. Oikos 82:212–222
Bonan GB (2008) Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449
Bortier K, Ceulemans R, de Temmerman L (2000) Effects of tropospheric ozone on woody plants. In: Agrawal SB, Agrawal M (eds) Environmental pollution and plant responses. CRC Press, Boca Raton, pp 153–182
Cabané M, Pireaux J-C, Léger E, Weber E, Dizengremel P, Pollet B, Lapierre C (2004) Condensed lignins are synthesized in poplar leaves exposed to ozone. Plant Physiol 134:586–594
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics—protection from photodamage not herbivores. Oikos 99:166–172
Couture JJ (2011) Impact of elevated CO2 and O3 on community herbivory in a northern temperate forest (Ph.D. dissertation). University of Wisconsin, Madison
Dickson RE, Lewin KF, Isebrands JG, Coleman MD, Heilman WE, Riemenschneider DE, Sober J, Host GE, Zak DR, Pregitzer KS, Karnosky DF (2000) Forest atmosphere carbon transfer storage II (FACTS II)—the Aspen Free-air CO2 and O3 Enrichment (FACE) Project: an overview (Gen Tech Rep NC-214). USDA Forest Service, North Central Research Station, Rhinelander
Donaldson JR, Stevens MT, Barnhill HR, Lindroth RL (2006) Age-related shifts in leaf chemistry of clonal aspen. J Chem Ecol 32:1415–1429
Fajvan MA, Wood JM (1996) Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. Forest Ecol Manag 89:79–88
Filion M, Dutilleul P, Potvin C (2000) Optimum experimental design for Free-Air Carbon dioxide Enrichment (FACE) studies. Glob Change Biol 6:843–854
Finzi A, Norby R, Carlo C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME, Ledford J, Liberloo M, Oren R, Polle A, Pritchard S, Zak DR, Schlesinger WH, Ceulemans R (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104:14014–14019
Fitzgerald TD (1995) The tent caterpillars. Cornell University Press, New York
Fowler D, Cape JN, Coyle M, Flechard C, Kuylenstierna J, Hicks K, Derwent D, Johnson C, Stevenson D (1999) The global exposure of forests to air pollutants. Water Air Soil Poll 116:5–32
Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel L (1997) High molecular weight polyphenolics (tannins) as antioxidants. J Agr Food Chem 46:1887–1892
Hättenschwiler S, Schafellner C (2004) Gypsy moth feeding in the canopy of a CO2-enriched mature forest. Glob Change Biol 10:1899–1908
Heath RL (2008) Modification of the biochemical pathways of plants induced by ozone: What are the varied routes to change? Enviro Pollut 155:453–463
Hemming JDC, Lindroth RL (1999) Effects of light and nutrient availability on aspen: growth, phytochemistry, and insect performance. J Chem Ecol 25:1687–1714
Holton MK, Lindroth RL, Nordheim EV (2003) Foliar quality influences tree-herbivore–parasitoid interactions: effects of elevated CO2, O3, and plant genotype. Oecologia 137:233–244
Honĕk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Hunter MD (2001) Effects of elevated atmospheric carbon dioxide on insect–plant interactions. Agric Forest Entomol 3:153–159
Intergovernmental Panel on Climate Change (IPCC) (2007) In: Solomon S et al. (eds) Climate Change 2007: the scientific basis (Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change). Cambridge University Press, New York
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264
Karnosky DF, Mankovska B, Percy K, Dickson RE, Podila GK, Sober J, Noormets A, Hendrey G, Coleman MD, Kubiske M, Pregitzer KS, Isebrands JG (1999) Effects of tropospheric O3 on trembling aspen and interaction with CO2: results from an O3-gradient and a FACE experiment. Water Air Soil Poll 116:311–332
Karnosky DF, Werner H, Holopainen T, Percy K, Oksanen T, Oksanen E, Heerdt C, Fabian P, Nagy J, Heilman W, Cox R, Nelson N, Matyssek R (2007) Free-air exposure systems to scale up ozone research to mature trees. Plant Biol 9:181–190
Kinney KK, Lindroth RL, Jung SM, Nordheim EV (1997) Effects of CO2 and NO3 availability on deciduous trees: phytochemistry and insect performance. Ecology 78:215–230
Knepp RG, Hamilton JG, Zangerl AR, Berenbaum MR, DeLucia EH (2007) Foliage of oaks grown under elevated CO2 reduces performance of Antheraea polyphemus (Lepidoptera: Saturniidae). Environ Entomol 36:609–617
Kopper BJ, Lindroth RL (2003a) Effects of elevated carbon dioxide and ozone on the phytochemistry of aspen and performance of an herbivore. Oecologia 134:95–103
Kopper BJ, Lindroth RL (2003b) Response of trembling aspen (Populus tremuloides) phytochemistry and aspen blotch leafminer (Phyllonorycter tremuloidiella) performance to elevated levels of atmospheric CO2 and O3. Agric Forest Entomol 5:17–26
Kopper BJ, Lindroth RL, Nordheim EV (2001) CO2 and O3 effects on paper birch (Betulaceae: Betula papyrifera) phytochemistry and whitemarked tussock moth (Lymantriidae: Orgyia leucostigma) performance. Environ Entomol 30:1119–1126
Körner C (2006) Plant CO2 responses: an issue of definition, time, and resources supply. New Phytol 172:393–411
Kubiske ME, Quinn VS, Marquardt PE, Karnosky DF (2007) Effects of elevated atmospheric CO2 and/or O3 on intra- and interspecific competitive ability of aspen. Plant Biol 9:342–355
Lavola A, Julkunen-Tiitto R, Paakkonen E (1994) Does ozone stress change the primary or secondary metabolites of birch (Betula pendula Roth)? New Phytol 126:637–642
Lincoln DE, Fajer ED, Johnson RH (1993) Plant–insect herbivore interactions in elevated CO2 environments. Trends Ecol Evol 8:64–68
Lindroth RL (1996a) CO2-mediated changes in tree chemistry and tree–Lepidoptera interactions. In: Koch GW, Mooney HA (eds) Carbon dioxide and terrestrial ecosystems. Academic, San Diego, pp 105–120
Lindroth RL (1996b) Consequences of elevated atmospheric CO2 for forest insects. In: Körner C, Bazzaz FA (eds) Carbon dioxide, populations, and communities. Academic, San Diego, pp 347–361
Lindroth RL (2010) Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J Chem Ecol 36:2–21
Lindroth RL, Hwang S-Y (1996) Diversity, redundancy, and multiplicity in chemical defense systems of aspen. In: Romero JT, Saunders JA, Barbosa P (eds) Recent advances in phytochemistry: phytochemical diversity and redundancy in ecological interactions. Plenum, New York, pp 26–51
Lindroth RL, Scriber JM, Hsia MTS (1988) Chemical ecology of the tiger swallowtail: mediation of host use by phenolic glycosides. Ecology 69:814–822
Lindroth RL, Kinney KK, Platz CP (1993) Responses of deciduous trees to elevated atmospheric CO2: productivity, phytochemistry, and insect performance. Ecology 74:763–777
Lindroth RL, Wood SA, Kopper BJ (2002) Responses of quaking aspen genotypes to enriched CO2: foliar chemistry and tussock moth performance. Agric Forest Entomol 4:315–323
Lovett GM, Canham CD, Arthur MA, Weathers KC, Fitzhugh RD (2006) Forest ecosystem response to exotic pests and pathogens in eastern North America. Bioscience 56:395–405
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Mattson WJ, Addy ND (1975) Phytophagous insects as regulators of forest primary production. Science 190:515–522
Mattson WJ, Herms DA, Witter JA, Allen DC (1991) Woody plant grazing systems: North American outbreak folivores and their host plants. In: Baranchikov YN, Mattson WJ, Hain FP, Payne TL (eds) Forest insect guilds: patterns of interactions with host trees (Gen Tech Rep NE-153). USDA Forest Service, North Central Research Station, Rhinelander
McGrath GM, Karnosky DF, Ainsworth EA (2010) Spring leaf flush in aspen (Populus tremuloides) clones is altered by long-term growth at elevated carbon dioxide and elevated ozone concentration. Environ Pollut 105:1023–1028
Oksanen EA (2003) Physiological responses of birch to ozone a comparison between open-soil-grown trees exposed for six growing seasons and potted seedlings exposed for one season. Tree Physiol 23:603–614
Osier TL, Hwang T-Y, Lindroth RL (2000) Effects of phytochemical variation in quaking aspen Populus tremuloides clones on gypsy moth Lymantria dispar performance in the field and laboratory. Ecol Entomol 25:197–207
Peltonen PA, Vapaavuori E, Heionen J, Julkunen-Tiitto R, Holopainen JK (2010) Do elevated atmospheric CO2 and O3 affect food quality and performance of folivorous insects on silver birch? Glob Change Biol 16:918–935
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223–230
Raubenheimer D, Simpson SJ (1992) Analysis of covariance: an alternative to nutritional indices. Entomol Exp Appl 62:221–231
Roth SK, Lindroth RL (1995) Elevated atmospheric CO2: effects on phytochemistry, insect performance and insect–parasitoid interactions. Glob Change Biol 1:173–182
Scriber JM, Slansky F (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211
Stiling P, Cornelissen T (2007) How does elevated carbon dioxide (CO2) affect plant–herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob Change Biol 13:1–20
Stireman JO III, Dyer LA, Janzen DH, Singer MS, Lill JT, Marquis RJ, Rickelfs RE, Gentry GL, Hallwachs W, Coley PD, Barone JA, Greeney HF, Connahas H, Barbosa P, Morais HC, Diniz IR (2005) Climatic unpredictability and caterpillar parasitism: implications of global warming. Proc Natl Acad Sci USA 102:17384–17387
United States Department of Agriculture, Forest Service (2003) Forest insect and disease conditions in the United States 2001. http://www.fs.fed.us/foresthealth/publications/ConditionsReport_01_final.pdf
United States Department of Agriculture, Forest Service (2009) Forest insect and disease conditions in the United States 2008. http://www.fs.fed.us/foresthealth/publications/ConditionsReport_08_final.pdf
Valkama E, Koricheva J, Oksanen E (2007) Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Glob Change Biol 13:184–201
Vigue LM, Lindroth RL (2010) Effects of genotype, elevated CO2 and elevated O3 on aspen phytochemistry and leaf beetle Chrysomela crotchi performance. Agric Forest Entomol 12:267–276
Williams RS, Lincoln DE, Norby RJ (2003) Development of gypsy moth larvae feeding on red maple saplings at elevated CO2 and temperature. Oecologia 137:114–122
Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP (2009) Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Glob Change Biol 15:396–424
Wold S, Ruhe A, Wold H, Dunn WJ (1984) The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J Sci Stat Comp 5:735–743
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 58:109–130
Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Glob Change Biol 12:27–41
Acknowledgments
We are grateful to T.D. Fitzgerald for generously providing forest tent caterpillar egg masses. We thank K.F. Rubert-Nason for assistance with the chemical analysis of phenolic glycosides, and M. Bushell for laboratory assistance. We also thank P.A. Townsend, P.T. Wolter, and S.P. Serbin for assistance with PLSR analysis. One reviewer provided particularly constructive comments on the manuscript. Aspen FACE was principally supported by the Office of Science (BER), U.S. Department of Energy, grant no. DE-FG02-95ER62125 to Michigan Technological University, and contract no. DE-AC02-98CH10886 to Brookhaven National Laboratory, the US Forest Service Northern Global Change Program and North Central Research Station, Michigan Technological University, and Natural Resources Canada—Canadian Forest Service. This work was supported by the U.S. Department of Energy (Office of Science, BER), grant DE-FG02-06ER64232, to RL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Couture, J.J., Meehan, T.D. & Lindroth, R.L. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 168, 863–876 (2012). https://doi.org/10.1007/s00442-011-2139-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2139-1