Abstract
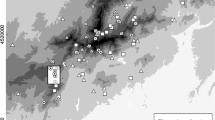
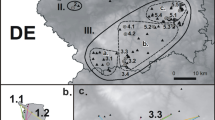
High mountain ecosystems are extreme habitats for all organisms and therefore demand specific adaptations. In this context, we studied the ecology of the butterfly Euphydryas aurinia debilis in the High Tauern (Austria) and compared the obtained data against the ecology of the species in lower elevation habitats. We performed mark-release-recapture studies over the entire flight periods (end of June to end of July) in 2007 and 2008 to analyse the fundamental ecological parameters of a population. The demography of males and females was similar in both years, and no indication of typical protandry was detected. We observed a generally low dispersal of the individuals in both years, but males dispersed significantly more than females in 2008; this finding of low vagility was supported by allozyme analyses. Furthermore, butterflies survived periods of several days of continuously closed snow cover without any indication of increased mortality rates. In these three traits, this alpine population of E. aurinia apparently has ecological and physiological adaptations to the extreme requirements of high-altitude habitats and strongly deviates from the lower elevation populations.


Similar content being viewed by others
References
Altshuler D, Dudley R (2006) Adaptations to life at high elevation: an introduction to the symposium. Integr Comp Biol 46:3–4
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S (2001) The millennium atlas of butterflies in Britain and Ireland. Oxford University Press, Oxford
Baguette M (2003) Long distance dispersal and landscape occupancy in a metapopulation of the cranberry fritillary butterfly. Ecography 26:153–160
Baguette M, Schtickzelle N (2006) Negative relationship between dispersal distance and demography in butterfly metapopulations. Ecology 87:648–654
Bale JS (1987) Insect cold hardiness: freezing and supercooling—an ecophysiological perspective. J Insect Physiol 33:899–908
Baust JG, Rojas RR (1985) Review—insect cold hardiness: facts and fancy. J Insect Physiol 31:755–759
Berner D, Körner C, Blanckenhorn W (2004) Grasshopper populations across 2000 m of altitude: is there life history adaptation? Ecography 27:733–740
Besold J, Schmitt T, Tammaru T, Cassel-Lundhagen A (2008) Strong genetic impoverishment from the centre of distribution in southern Europe to peripheral Baltic and isolated Scandinavian populations of the pearly heath butterfly. J Biogeogr 35:2090–2101
Betzholtz PE, Ehrig A, Lindeborg M, Dinnétz P (2007) Food plant density, patch isolation and vegetation height determine occurrence in a Swedish metapopulation of the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae). J Insect Conserv 11:343–350
Blanckenhorn W (1997) Altitudinal life history variation in the dung flies Scathophaga stercoraria and Sepsis cynipsea. Oecologia 1009:342–352
Chappel M (1983) Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia 56:126-131
Cooch E, White G (2007) Program MARK. A gentle introduction, 6th edn. Internet-Document. http://www.phidot.org/software/mark/docs/book/
Cullenward MJ, Ehrlich PR, White RR, Holdren CE (1979) The ecology and population genetics of an alpine checkerspot butterfly, Euphydryas anicia. Oecologia 38:1–12
Dillon ME, Frazier MR, Dudley R (2005) Into thin air: physiology and evolution of alpine insects. Integr Comp Biol 46:49–61
Dingle H, Mousseau T (1994) Geographic variation in embryonic development time and stage of diapause in a grasshopper. Oecologia 97:179–185
Dingle H, Mousseau T, Scott S (1990) Altitudinal variation in life cycle syndromes of California populations of the grasshopper Melanoplus sanguinipes (F.). Oecologia 84:199–206
Duman JG (2001) Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol 63:327–357
Excoffier L, Laval G, Schneider S (2005) Arlequin Ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Felsenstein J (1993) PHYLIP (phylogeny inference package) Ver. 3.5.c. Department of Genetics, University of Washington, Seattle
Fowles AP, Smith RG (2006) Mapping the habitat quality of patch networks for the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae) in Wales. J Insect Conserv 10:161–177
Fric Z, Konvicka M (2007) Dispersal kernels of butterflies: power-law functions are invariant to marking frequency. Basic Appl Ecol 8:377–386
Habel J, Schmitt T, Müller P (2005) The fourth paradigm pattern of post-glacial range expansion of European terrestrial species: the phylogeography of the marbled white butterfly (Satyrinae, Lepidoptera). J Biogeogr 32:1489–1497
Habel J, Meyer M, Mousadik A, Schmitt T (2008) Africa goes Europe: the complete phylogeography of the Marbled White butterfly species complex Melanargia galathea/M. lachesis (Lepidoptera: Satyridae). Org Divers Evol 8:121–129
Harris H, Hopkinson DA (1978) Handbook of enzyme electrophoresis in human genetics. North-Holland Publishers, Amsterdam
Hebert PDN, Beaton MJ (1993) Methodologies for allozyme analysis using cellulose acetate electrophoresis. Helena Laboratories, Beaumont, TX
Hovestadt T, Nieminen M (2009) Costs and benefits of dispersal in butterflies. In: Settele J, Shreeve T, Konvicka M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 97–106
Hula V, Konvicka M, Pavlicko A, Zdenek F (2004) Marsh Fritillary (Euphydryas aurinia) in the Czech Republic: monitoring, metapopulation structure, and conservation of an endangered butterfly. Entomol Fenn 15:231–241
Joyce DA, Pullin AS (2003) Conservation implications of the distribution of the genetic diversity at different scales: a case study using the marsh fritillary butterfly (Euphydryas aurinia). Biol Conserv 114:453–461
Junker M, Schmitt T (2010) Demography, dispersal and movement pattern of Euphydryas aurinia (Lepidoptera: Nymphalidae) at the Iberian Peninsula: an alarming example in an increasingly fragmented landscape? J Insect Conserv 14:237–246
Kadlec T, Vrba P, Kepka P, Schmitt T, Konvicka M (2010) Tracking the decline of once-common butterfly: delayed oviposition, demography and population genetics in the Hermit, Chazara briseis. Anim Conserv 13:172–183
Keyghobadi N, Roland J, Strobeck C (1999) Influence of landscape on the population genetic structure of the alpine butterfly Parnassius smintheus (Papilionodae). Mol Ecol 8:1481–1495
Konvicka M, Hula V, Fric Z (2003) Habitat of pre-hibernating larvae of the endangered butterfly Euphydryas aurinia (Lepidoptera: Nymphalidae): what can be learned from vegetation composition and architecture? Eur J Entomol 100:313–322
Konvicka M, Novak J, Benes J, Fric Z, Bradley J, Keil P, Hrcek J, Chobot K, Marhoul P (2008) The last population of the Woodland Brown butterfly (Lopinga achine) in the Czech Republic: habitat use, demography and site management. J Insect Conserv 12:549–560
Kudrna O (1986) Butterflies of Europe, vol 8. Aspects of the conservation of butterflies in Europe. Aula, Wiesbaden
Kuras T, Benes J, Fric Z, Konvicka M (2003) Dispersal patterns of endemic alpine butterflies with contrasting population structures: Erebia epiphron and E. sudetica. Pop Ecol 45:115–123
Mani MS (1968) Ecology and biogeography of high altitude insects. Ser Entomol 4:530
Miaud C, Merilä J (2000) Local adaptation or environmental induction? Causes of population differentiation in alpine amphibians. Biota 2:31–50
Müller M (1987) Handbuch ausgewählter Klimastationen der Erde. Universität Trier, Paulinus-Druckerei, Trier
Munguira ML, Martin J, García-Barros E, Viejo JL (1997) Use of space and resources in a Mediterranean population of the butterfly Euphydryas aurinia. Acta Oecol 18:597–612
Nei M (1972) Genetic distances between populations. Am Nat 106:283–291
Nève G (2009) Population genetics of butterflies. In: Settele J, Shreeve T, Konvicka M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 107–129
Nève G, Singer MC (2008) Protandry and postandry in two related butterflies: conflicting evidence about sex-specific trade-offs between adult size and emergence time. Evol Ecol 22:701–709
Nowicki P, Bonelli S, Barbero F, Balletto E (2009) Relative importance of density-dependent regulation and environmental stochasticity for butterfly population dynamics. Oecologia 161:227–239
Peñuelas J, Sardans J, Stefanescu C, Parella T, Filella I (2006) Lonicera implexa leaves bearing naturally laid eggs of the specialist herbivore Euphydryas aurinia have dramatically greater concentrations of iridoid glycosides than other leaves. J Chem Ecol 32:1925–1933
Richardson BJ, Baverstock PR, Adams M (1986) Allozyme electrophoresis. A handbook for animal systematics and population studies. Academic Press, San Diego
Roff D (1980) Optimizing development time in a seasonal environment: the “ups and downs” of clinal variation. Oecologia 45:202–208
Roland J, Keyghobadi N, Fownes S (2000) Alpine Parnassius butterfly dispersal: effects of landscape and population size. Ecology 81:1642–1653
Schmitt T, Besold J (2010) Upslope movements and large scale expansions: the taxonomy and biogeography of the Coenonympha arcania–darwiniana–gardetta butterfly species complex. Zool J Linn Soc Lond (in press)
Schmitt T, Haubrich K (2008) The genetic structure of the mountain forest butterfly Erebia euryale unravels the late Pleistocene and postglacial history of the mountain coniferous forest biome in Europe. Mol Ecol 17:2194–2207
Schmitt T, Röber S, Seitz A (2005) Is the last glaciation the only relevant event for the present genetic population structure of the meadow brown butterfly Maniola jurtina (Lepidoptera: Nymphalidae)? Biol J Linn Soc 85:419–431
Schmitt T, Hewitt GM, Müller P (2006) Disjunct distributions during glacial and interglacial periods in mountain butterflies: Erebia epiphron as an example. J Evol Biol 19:108–113
Schtickzelle N, Baguette M, Le Boulengé É (2003) Modelling insect demography from capture–recapture data: comparison between the constrained linear models and the Jolly–Seber analytical method. Can Entomol 135:313–323
Schtickzelle N, Choutt J, Goffart P, Fichefet V, Baguette M (2005) Metapopulation dynamics and conservation of the marsh fritillary butterfly: population viability analysis and management options for a critically endangered species in Western Europe. Biol Conserv 126:569–581
Schweizer Bund für Naturschutz (1987) Tagfalter und ihre Lebensräume. Basel
Siegismund HR (1993) G-Stat, ver. 3. Genetical statistical programs for the analysis of population data. The Arboretum, Royal Veterinary and Agricultural University, Horsholm, Denmark
Sinclair BJ, Vernon P, Klok CJ, Chown SL (2003a) Insects at low temperatures: an ecological perspective. Trends Ecol Evol 18:257–262
Sinclair BJ, Addo-Bediako A, Chown SL (2003b) Climatic variability and the evolution of insect freeze tolerance. Biol Rev 78:181–195
Singer MC, Wee B (2005) Spatial pattern in checkerspot butterfly-host plant association at local, metapopulation and regional scales. Ann Zool Fenn 42:347–361
Somme L (2008) Adaptations of terrestrial arthropods to the alpine environment. Biol Rev 4:367–407
Stefanescu C, Peñuelas J, Sardan J, Filella I (2006) Females of the specialist butterfly Euphydryas aurinia (Lepidoptera: Nymphalidae: Melitaeini) select the greenest leaves of Lonicera implexa (Caprifoliaceae) for oviposition. Eur J Entomol 103:569–574
Wang R, Wang Y, Chen J, Lei G, Xu R (2004) Contrasting movement patterns in two species of chequerspot butterflies, Euphydryas aurinia and Melitaea phoebe, in the same patch network. Ecol Entomol 29:367–374
Warren MS, Munguira ML, Ferrin J (1994) Notes on the distribution, habitats and conservation of Euphydryas aurinia Rottemburg Lepidoptera: Nymphalidae in Spain. Entomol Gaz 45:5–12
Zimmermann K, Fric Z, Filipová L, Konvicka M (2005) Adult demography, dispersal and behaviour of Brenthis ino (Lepidoptera: Nymphalidae): how to be a successful wetland butterfly. Eur J Entomol 102:699–706
Acknowledgments
We acknowledge the DFG for financing the scholarship of Marius Junker in the graduate school (Verbesserung von Normsetzung und Normanwendung im integrierten Umweltschutz durch rechts- und naturwissenschaftliche Kooperation; no. 1319) at the University of Trier, and the Hohe Tauern National Park for the permission to perform our studies within the national park. Furthermore, we thank Großglockner Hochalpenstraße for financial support of the genetic analyses and for the permission to freely use the alpine road, and the Haus der Natur, Museum für Natur und Technik for accommodation in the Wilfried Haslauer Haus research station during the field studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Junker, M., Wagner, S., Gros, P. et al. Changing demography and dispersal behaviour: ecological adaptations in an alpine butterfly. Oecologia 164, 971–980 (2010). https://doi.org/10.1007/s00442-010-1720-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1720-3