Abstract
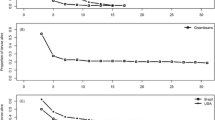
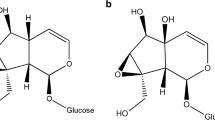
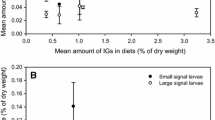
Host use by herbivores is largely determined by host properties such as nutrient content and chemical defence against foragers. The impacts of these attributes on a herbivore may largely depend on its life cycle stage. Lichen species are known to differ in nutritional quality and level of chemical defence and, consequently, vary as fodder for herbivores. The aim of this study was to explore the impact of several lichen species and the presence of their secondary metabolites on their use as hosts by a specialist lichen-feeder, Cleorodes lichenaria. This study also addressed, for the first time, how a specialist lichen-feeder deals with different lichen secondary metabolites. In the beginning of their development, larvae grew better on Xanthoria parietina than on the other host lichens, whereas older larvae grew best on Ramalina fraxinea. Lichen secondary chemicals in R. fraxinea and Parmelia sulcata hindered larval growth in the beginning but after 75 days lichen secondary chemicals had no impact on the mass of larvae. Physodic acids in Hypogymnia physodes were lethal to larvae. In general, larvae metabolized 70–95% of ingested lichen secondary chemicals and the rest of these were excreted in frass. Lichen secondary metabolites in P. sulcata restrict and in H. physodes prevent their use as a host for C. lichenaria larvae. Our main finding, the ability of larvae to metabolize several lichen secondary metabolites, indicates digestive adaptation to these chemicals. No signs of sequestration of these chemicals were found.




Similar content being viewed by others
References
Agrawal AA, Kurashige NS (2003) A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol 29:1403–1416
Backor M, Dvorsky K, Fahselt D (2003) Influence of invertebrate feeding on the lichen Cladonia pocillum. Symbiosis 34:281–291
Becerra JX (1997) Insects on plants: macroevolutionary chemical trends in host use. Science 276:253–256
Berenbaum MR, Zangerl AR (1993) Furanocoumarin metabolism in Papilio polyxenes: biochemistry, genetic variability, and ecological significance. Oecologia 95:370–375
Berenbaum MR, Zangerl AR, Lee K (1989) Chemical barriers to adaptation by a specialist herbivore. Oecologia 80:501–506
Bernays EA, Chapmann RF (1994) Host-plant selection by phytophagous insects. Chapmann & Hall, New York
Braby MF (1994) The significance of egg size variation in butterflies in relation to hostplant quality. Oikos 71:119–129
Calcagno MP, Coll J, Lloria J, Faini F, Alonso-Amelot ME (2002) Evaluation of synergism in the feeding deterrence of some furanocoumarins on Spodoptera littoralis. J Chem Ecol 28:175–194
Cocchietto M, Skert N, Nimis PF, Sava G (2002) A review on usnic acid, an interesting natural compound. Naturwissenschaften 89:137–146
Crawley MJ, Nachapong M (1984) Facultative defences and specialist herbivores? Cinnabar moth (Tyria jacobaeae) on the regrowth foliage of ragwort (Senecio jacobaea). Ecol Entomol 9:389–393
Culberson CF, Culberson WL, Johnson A (1977) Second supplement to chemical and botanical guide to lichen products. The American Bryological and Lichenological Society, Missouri Botanical Garden, St. Louis
Dyer LA, Dodson CD, Stireman JO III, Tobler MA, Smilanich AM, Fincher RM, Letourneau DK (2003) Synergistic effects of three piper amides on generalist and specialist herbivores. J Chem Ecol 29:2499–2544
Dzubaj A, Backor M, Tomko J, Peli E, Tuba Z (2008) Tolerance of the lichen Xanthoria parietina (L.) th. fr. to metal stress. Ecotoxicol Environ Saf 70:319–326
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Elix JA (1996) Biochemistry and secondary metabolites. In: Thomas JN (ed) Lichen biology. Cambridge University Press, Cambridge, pp 154–180
Emmerich R, Giez I, Lange OL, Proksch P (1993) Toxicity and antifeedant activity of lichen compounds against the polyphagous herbivorous insect Spodoptera littoralis. Phytochemistry 33:1389–1394
Fahselt D (1994) Secondary biochemistry of lichens. Symbiosis 16:117–165
Farrell B, Mitter C (1990) Phylogenesis of insect/plant interactions: have Phyllobrotica leaf beetles (Chrysomelidae) and the lamiales diversified in parallel? Evolution 44:1389–1403
Farrell BD, Mitter C (1998) The timing of insect/plant diversification: Might tetraopes (Coleoptera: Cerambycidae) and asclepias (Asclepiadaceae) have co-evolved? Biol J Linn Soc 63:553–577
Feige GB, Lumbsch HT, Huneck S, Elix JA (1993) Identification of lichen substances by a standardized high-performance liquid chromatographic method. J Chromatogr 646:417–427
Friedl T, Büdel B (1996) Photobionts. In: Nash TH (ed) Lichen biology. Cambridge University Press, Cambridge, pp 8–23
Gauslaa Y (2005) Lichen palatability depends on investments in herbivore defence. Oecologia 143:94–105
Giez I, Lange OL, Proksch P (1994) Growth retarding activity of lichen substances against the polyphagous herbivorous insect Spodoptera littoralis. Biochem Syst Ecol 22:113–120
Glendinning JI (2002) How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol Exp Appl 104:15–25
Hägele BF, Rowell-Rahier M (2000) Choice, performance and heritability of performance of specialist and generalist insect herbivores towards cacalol and seneciphylline, two allelochemicals of Adenostyles alpina (Asteraceae). J Evol Biol 13:131–142
Hesbacher S, Giez I, Embacher G, Fielder K, Max W, Trawoger A, Turk R, Lange OL, Proksch P (1995) Sequestration of lichen compounds by lichen-feeding members of the arctiidae (Lepidoptera). J Chem Ecol 21:2079–2089
Hwang S, Hwang F, Shen T (2007) Shifts in developmental diet breadth of Lymantria xylina (Lepidoptera: Lymantriidae). J Econ Entomol 100:1166–1172
Hyvärinen M, Koopmann R, Hormi O, Tuomi J (2000) Phenols in reproductive and somatic structures of lichens: a case of optimal defence? Oikos 91:371–375
Ingolfsdottir K (2002) Usnic acid. Phytochemistry 61:729–736
Johnson KS, Scriber JM, Nair M (1996) Phenylpropenoid phenolics in sweetbay magnolia as chemical determinants of host use in saturniid silkmoths (Callosamia). J Chem Ecol 22:1955–1970
Karban R, Agrawal AA (2002) Herbivore offense. Annu Rev Ecol Syst 33:641–664
Krischik VA, Goth RW, Barbosa P (1991) Generalized plant defense: effects on multiple species. Oecologia 85:562–571
Landau I, Mueller-Schaerer H, Ward PI (1994) Influence of cnicin, a sesquiterpene lactone of Centaurea maculosa (asteraceae), on specialist and generalist insect herbivores. J Chem Ecol 20:929–942
Lawrey LD (1983) Lichen herbivore preference: a test of two hypotheses. Am J Bot 70:1188–1194
Lawrey JD (1984) Biology of lichenized fungi. Praeger, New York
Lawrey JD (1989) Lichen secondary compounds: evidence for a correspondence between antiherbivore and antimicrobial function. Bryologist 92:326–328
Lumbsch HT (2002) Analysis of phenolic products in lichens for identification and taxonomy. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology: culturing, biochemistry, ecophysiology, and use in biomonitoring. Springer, Berlin, pp 281–295
Macel M, Klinkhamer PI, Vrieling K, van der Meijden E (2002) Diversity of pyrrolizidine alkaloids in Senecio species does not affect the specialist herbivore Tyria jacobaeae. Oecologia 133:541–550
Mikkola K, Jalas I, Peltonen O (1989) Suomen perhoset. mittarit 2. Suomen perhostutkijain seura. Recallmed Oy, Hanko
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
Orange A, James PW, White FJ (2001) Microchemical methods for the identification of lichens. British Lichen Society, London
Pöykkö H (2006) Females and larvae of a geometrid moth, Cleorodes lichenaria, prefer a lichen host that assures shortest larval period. Environ Entomol 35:1669–1676
Pöykkö H (2009) Egg maturation and oviposition strategy of a capital breeder, Cleorodes lichenaria, feeding on lichens at the larval stage. Ecol Entomol 34:254–261
Pöykkö H, Hyvärinen M (2003) Host preference and performance of lichenivorous Eilema spp. larvae in relation to lichen secondary metabolites. J Anim Ecol 72:383–390
Pöykkö H, Hyvärinen M, Bačkor M (2005) Removal of lichen secondary metabolites affects food choice and survival of lichenivorous moth larvae. Ecology 86:2623–2632
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rawlins JE (1984) Mycophagy in lepidoptera. In: Wheeler Q, Blackwell M (eds) Fungus–insect relationships. Perspectives in ecology and evolution. Columbia University Press, New York, pp 382–423
Schoonhoven LM, van Loon JJA, Dicke M (2004) Insect–plant biology, 2nd edn. Oxford University Press, New York
Scott IM, Puniani E, Durst T, Phelps D, Merali S, Assabgui RA, Sanchez-Vindas P, Poveda L, Philogene BJR, Arnason JT (2002) Insecticidal activity of Piper tuberculatum jacq. extracts: synergistic interaction of piperamides. Agric For Entomol 4:137–144
Slansky F Jr (1979) Effect of the lichen chemicals atranorin and vulpinic acid upon feeding and growth of larvae of the yellow-striped armyworm, Spodoptera ornithogallii. Environ Entomol 8:865–868
Solhaug KA, Gauslaa Y (1996) Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 108:412–418
Solhaug KA, Gauslaa Y (2001) Acetone rinsing—a method for testing ecological and physiological roles of secondary compounds in living lichens. Symbiosis 30:301–315
Stocker-Wörgötter E, Elix JA, Grube M (2004) Secondary chemistry of lichen-forming fungi: chemosyndromic variation and DNA-analyses of cultures and chemotypes in the Ramalina farinacea complex. Bryologist 107:152–162
Tay T, Turk AO, Yilmaz M, Turk H, Kivanc M (2004) Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea and its (+)-usnic acid, norstictic acid, and protocetraric acid constituents. Zeitschr Naturforsch C-J Biosci 59:384–388
Wahlberg N (2001) The phylogenetics and biochemistry of host-plant specialization in melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution 55:522–537
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behavior of first instar larval lepidoptera. Annu Rev Entomol 47:361–393
Zovi D, Stastny M, Battisti A, Larsson S (2008) Ecological costs on local adaptation of an insect herbivore imposed by host plants and enemies. Ecology 89:1388–1398
Acknowledgments
This study was financially supported by the Faculty of Natural Sciences, University of Oulu, Finland, the Finnish Cultural Foundation and by the Slovak Grant Agency (VEGA 1/4337/07). Mr Mark Goodall has kindly revised the English language of the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Hoffmeister.
Rights and permissions
About this article
Cite this article
Pöykkö, H., Bačkor, M., Bencúrová, E. et al. Host use of a specialist lichen-feeder: dealing with lichen secondary metabolites. Oecologia 164, 423–430 (2010). https://doi.org/10.1007/s00442-010-1682-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1682-5