Abstract
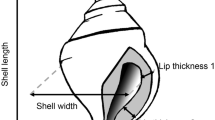
Reliable cues that communicate current or future environmental conditions are a requirement for the evolution of adaptive phenotypic plasticity, yet we often do not know which cues are responsible for the induction of particular plastic phenotypes. I examined the single and combined effects of cues from damaged prey and predator cues on the induction of plastic shell defenses and somatic growth in the marine snail Nucella lamellosa. Snails were exposed to chemical risk cues from a factorial combination of damaged prey presented in isolation or consumed by predatory crabs (Cancer productus). Water-borne cues from damaged conspecific and heterospecific snails did not affect plastic shell defenses (shell mass, shell thickness and apertural teeth) or somatic growth in N. lamellosa. Cues released by feeding crabs, independent of prey cue, had significant effects on shell mass and somatic growth, but only crabs consuming conspecific snails induced the full suite of plastic shell defenses in N. lamellosa and induced the greatest response in all shell traits and somatic growth. Thus the relationship between risk cue and inducible morphological defense is dependent on which cues and which morphological traits are examined. Results indicate that cues from damaged conspecifics alone do not trigger a response, but, in combination with predator cues, act to signal predation risk and trigger inducible defenses in this species. This ability to “label” predators as dangerous may decrease predator avoidance costs and highlights the importance of the feeding habits of predators on the expression of inducible defenses.


Similar content being viewed by others
References
Alexander JE, Covich AP (1991) Predator avoidance by the fresh-water snail Physella virgata in response to the crayfish Procambarus simulans. Oecologia 87:435–442
Appleton RD, Palmer AR (1988) Water-borne stimuli released by predatory crabs and damaged prey induce more predator-resistant shells in a marine gastropod. Proc Natl Acad Sci USA 85:4387–4391
Avery R, Etter RJ (2006) Microstructural differences in the reinforcement of a gastropod shell against predation. Mar Ecol Prog Ser 323:159–170
Bamber SD, Naylor E (1997) Sites of release of putative sex pheromone and sexual behaviour in female Carcinus maenas (Crustacea: Decapoda). Estuar Coast Shelf Sci 44:195–202
Benard MF (2006) Survival trade-offs between two predator-induced phenotypes in Pacific treefrogs (Pseudacris regilla). Ecology 87:340–346
Bertness MD (1977) Behavioral and ecological aspects of shore-level size gradients in Thais lamellosa and Thais emarginata. Ecology 58:86–97
Bourdeau PE (2009a) Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90:1659–1669
Bourdeau PE (2009b) An inducible morphological defence is a passive by-product of behaviour in a marine snail. Proc R Soc B. doi:10.1098/rspb.2009.1295
Brown GE, Dreier VM (2002) Predator inspection behaviour and attack cone avoidance in a characin fish: the effects of predator diet and prey experience. Anim Behav 63:1175–1181
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5:338–352
Chivers DP, Wisenden BD, Smith RJF (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129
Conover WJ, Iman RL (1982) Analysis of covariance using the ran transformation. Biometrics 38:715–724
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Engqvist L (2005) The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav 70:967–971
Freeman AS (2007) Specificity of induced defenses in Mytilus edulis and asymmetrical predator deterrence. Mar Ecol Prog Ser 334:145–153
Griffiths CL, Richardson CA (2006) Chemically induced predator avoidance behaviour in the burrowing bivalve Macoma balthica. J Exp Mar Biol Ecol 331:91–98
Hagen NT, Andersen A, Stabell OB (2002) Alarm responses of the green sea urchin, Strongylocentrotus droebachiensis, induced by chemically labelled durophagous predators and simulated acts of predation. Mar Biol 140:365–374
Hardege JD et al (2002) Novel behavioural assay and partial purification of a female-derived sex pheromone in Carcinus maenas. Mar Ecol Prog Ser 244:179–189
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Hendrix LJ, Carter MW, Scott DT (1982) Covariance analyses with heterogeneity of slopes in fixed models. Biometrics 38:641–650
Huitema BE (1980) The analysis of covariance and alternatives. Wiley, New York
Jacobsen HP, Stabell OB (1999) Predator-induced alarm responses in the common periwinkle, Littorina littorea: dependence on season, light conditions, and chemical labelling of predators. Mar Biol 134:551–557
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signalling and predator labelling in the avoidance response of a marine gastropod. Oikos 104:43–50
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kristensen EA, Closs GP (2004) Anti-predator response of naive and experienced common bully to chemical alarm cues. J Fish Biol 64:643–652
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Can J Zool 68:619–640
Lively CM (1986) Canalization versus developmental conversion in a spatially-variable environment. Am Nat 128:561–572
McCollum SA, VanBuskirk J (1996) Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50:583–593
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692
Moran NA (1992) The evolutionary maintenance of alternative phenotypes. Am Nat 139:971–989
Palmer AR (1982) Growth in marine gastropods—a non-destructive technique for independently measuring shell and body-weight. Malacologia 23:63–73
Quinn GP, Keough MJ (2002) Experimental design and data analysis. Cambridge University Press, New York
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Rittschof D, Cohen JH (2004) Crustacean peptide and peptide-like pheromones and kairomones. Peptides 25:1503–1516
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Sih A (1987) Predators and prey lifestyles: an evolutionary and ecological overview. In: Kerfoot WC, Sih A (eds) Predation: direct and indirect impacts on aquatic communities. University Press of New England, London, pp 203–224
Slusarczyk M (1999) Predator-induced diapause in Daphnia magna may require two chemical cues. Oecologia 119:159–165
Smee DL, Weissburg MJ (2006) Hard clams (Mercenaria mercenaria) evaluate predation risk using chemical signals from predators and injured conspecifics. J Chem Ecol 32:605–619
Smith ME, Belk MC (2001) Risk assessment in western mosquitofish (Gambusia affinis): do multiple cues have additive effects? Behav Ecol Sociobiol 51:101–107
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Stabell OB, Ogbebo F, Primicerio R (2003) Inducible defences in Daphnia depend on latent alarm signals from conspecific prey activated in predators. Chem Senses 28:141–153
Taylor HH, Taylor EW (1991) The dorsoventral muscles of Carcinus maenas—evidence for hydrostatic-pressure control in a crab. Physiol Zool 64:1110–1129
Teplitsky C, Plenet S, Joly P (2004) Hierarchical responses of tadpoles to multiple predators. Ecology 85:2888–2894
Tollrian R, Harvell CD (eds) (1998) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Trussell GC, Nicklin MO (2002) Cue sensitivity, inducible defense, and trade-offs in a marine snail. Ecology 83:1635–1647
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245
Turner AM, Bernot RJ, Boes CM (2000) Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88:148–158
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton
Verschoor AM, Vos M, van der Stap I (2004) Inducible defences prevent strong population fluctuations in bi- and tritrophic food chains. Ecol Lett 7:1143–1148
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York
Yamada SB, Boulding EG (1996) The role of highly mobile crab predators in the intertidal zonation of their gastropod prey. J Exp Mar Biol Ecol 204:59–83
Acknowledgments
I would like to thank the director and staff of Friday Harbor Laboratories for logistical support. I am especially grateful to Megan Mach for help designing the experimental aquaria and assistance with daily maintenance of the experiment. Dianna Padilla, Geerat Vermeij, Jeff Levinton, Massimo Pigliucci, Geoff Trussell and three anonymous reviewers made constructive comments on earlier versions of the manuscript. Special thanks to Emily Thompson for her confident and judicious editing. A Ruth and Stephen Wainwright Fellowship funded this research. This is contribution number 1191 from the Department of Ecology and Evolution at Stony Brook University. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Geoffrey Trussell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bourdeau, P.E. Cue reliability, risk sensitivity and inducible morphological defense in a marine snail. Oecologia 162, 987–994 (2010). https://doi.org/10.1007/s00442-009-1488-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1488-5