Abstract
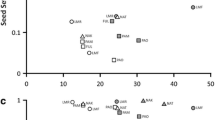
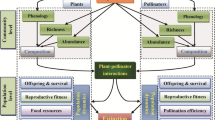
Few studies of plant–pollinator interactions in fragmented landscapes evaluate the consequences of floral visitor variation on multiple stages of plant reproduction. Given that fragmentation potentially has positive or negative effects on different organisms, and that self-incompatible plant species depend on pollinators for sexual reproduction, differences in floral visitor assemblages may affect certain plant reproductive stages. We evaluated how pollinator assemblage, availability of floral resources, pollination, reproductive output, and seed and seedling performance of Psychotria suterella Muell. Arg. varied among three fragmentation categories: non-fragmented habitats, fragments connected by corridors, and isolated fragments. Richness and frequency of floral visitors were greater in fragments than in non-fragmented sites, resulting mainly from the addition of species typically found in disturbed areas. Although 24 species visited Psychotria suterella flowers, bumblebees were considered the most important pollinators, because they showed the highest frequency of visits and were present in eight out of ten sites. Additionally, the number of pollen tubes per flower per visit was lower in areas without bumblebees. The increased visitation in fragments seemed to enhance pollination slightly. However, fruit and seed output, germination, and seed and seedling mass were similar in non-fragmented sites, connected sites, and isolated fragments. Our results suggested that, even for a self-incompatible species, responses to habitat fragmentation at different stages of plant reproduction might be decoupled from the responses observed in floral visitors, if fruit set is not pollen limited. If all reproductive stages were considered, variation on the small scale was more important than the variation explained by fragmentation category. In spite of its self-incompatible breeding system, this plant–pollinator system showed resilience to habitat fragmentation, mainly as a result of high availability of potential mates to P. suterella individuals, absence of pollen limitation, and the presence of bumblebees (Bombus spp.) throughout this highly connected landscape.





Similar content being viewed by others
References
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9:968–980
Aizen MA, Feinsinger P (1994a) Habitat fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 77:1779–1791
Aizen MA, Feinsinger P (1994b) Habitat fragmentation, native insect pollinators, and feral honeybees in Argentine “Chaco Serrano”. Ecol Appl 4:378–392
Alves LF, Metzger JP (2006) A regeneração florestal em áreas de floresta secundária na Reserva Florestal do Morro Grande, Cotia, SP. Biota Neotropica 6 (2), http://www.biotaneotropica.org.br/v6n2/pt/abstract?article+bn00606022006
Bhattacharya M, Primack RB, Gerwein J (2003) Are roads and railroads barriers to bumblebee movement in a temperate suburban conservation area? Biol Conserv 109:37–45
Bronstein JL (1995) The plant–pollinator landscape. In: Hansson L, Fahrig L, Merrian G (eds) Mosaic landscapes and ecological processes. Chapman and Hall, London, pp 256–288
Brown KS Jr (1992) Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Morellato LPC (ed) História Natural da Serra do Japi—ecologia e preservação de uma área florestal no sudeste do Brasil. Editora da Unicamp, Campinas, pp 143–186
Cairns CE, Villanueva-Gutiérrez R, Koptur S, Bray DB (2005) Bee populations, forest disturbance, and africanization in Mexico. Biotropica 37:686–692
Carvalho LMT, Fontes MA, Oliveira-Filho AT (2000) Tree species distribution in canopy gaps and mature forest in an area of cloud forest of the Ibitipoca Range, south-eastern Brazil. Plant Ecol 149:9–22
Checchia da Inês MC (2006) Fenologia e sucesso reprodutivo de Psychotria suterella (Rubiaceae): efeitos da disponibilidade de recursos e densidade floral. MS thesis, University of São Paulo, São Paulo
Crawley MT (2002) Statistical computing. An introduction to data analysis using S-plus. Wiley, Chichester
Cunningham SA (2000) Effects of habitat fragmentation on the reproductive ecology of four plant species in Mallee Woodland. Conserv Biol 14:758–768
Dean W (1995) With broadax and firebrand. The destruction of the Brazilian Atlantic forest. University of California Press, Berkeley
Duncan DH, Nicotra AB, Wood JT, Cunningham SA (2004) Plant isolation reduces outcross pollen receipt in a partially self-compatible herb. J Ecol 92:977–985
Fearnside PM (2005) Deforestation in Brazilian Amazonia: history, rates, and consequences. Conserv Biol 19:680–688
Grandisoli EAC (1997) Biologia reprodutiva e estrutura da população de Psychotria suterella Muell. Arg. (Rubiaceae) em um fragmento de mata secundária em São Paulo (SP). MS thesis, University of São Paulo, São Paulo
Hanski I (1998) Metapopulations dynamics. Nature 365:41–49
Harris LF, Johnson SD (2004) The consequences of habitat fragmentation for plant-pollinator mutualisms. Int J Trop Insect Sci 24:29–43
Herrerías-Diego Y, Quesada M, Stoner KE, Lobo JA (2006) Effects of forest fragmentation on phenological patterns and reproductive success of the tropical dry forest tree Ceiba aesculifolia. Conserv Biol 20:1111–1120
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Kareiva P, Wennergren U (1995) Connecting landscape to ecosystem and population processes. Nature 373:299–302
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Knight TM, Steets JA, Ashman T (2006) A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am J Bot 93:271–277
Kolb A (2005) Reduced reproductive successes and offspring survival in fragmented populations of the forest herb Phyteuma spicatum. J Ecol 93:1226–1237
Kunin WE (1997) Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J Ecol 85:225–234
Lopes LE, Buzato S (2005) Biologia reprodutiva de Psychotria suterella Muell. Arg. (Rubiaceae) e a abordagem de escalas ecológicas para a fenologia de floração e frutificação. Rev Bras Bot 28:785–795
Makino TT, Ohashi K, Sakai S (2007) How do floral display size and the density of surrounding flowers influence the likelihood of bumble bee revisitation to a plant? Func Ecol 21:87–95
Martensen AC, Develey PF, Metzger JP (2007) Effects of forest fragmentation on the understory bird community of the Atlantic Rainforest of southern Brazil. Ornitologia Neotropical (in press)
Matsumura C, Washitani I (2000) Effects of population size and pollinator limitation on seed-set of Primula sieboldii populations in a fragmented landscape. Ecol Res 15:307–322
Mavraganis K, Eckert G (2001) Effects of population size and isolation on reproductive output in Aquilegia canadensis (Ranunculaceae). Oikos 95:300–310
Murren CJ (2002) Effects of habitat fragmentation on pollination: pollinators, pollinia viability and reproductive success. J Ecol 90:100–107
Oliveira-Filho AT, Fontes MAL (2000) Patterns of floristic differentiation among Atlantic Forests in southeastern Brazil and influence of climate. Biotropica 32:793–810
Renner SS (1998) Effects of habitat fragmentation on plant-pollinator interactions in the tropics. In: Newbery DM, Prins HHT, Brown ND (eds) Dynamics of tropical communities. Blackwell, London, pp 339–360
Schemske DW, Pautler LP (1984) The effects of pollen composition on fitness components in a neotropical herb. Oecologia 62:31–36
Schulke B, Waser NM (2001) Long-distance pollinator flights and pollen dispersal between populations of Delphinium nuttallianum. Oecologia 127:239–245
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432
Stouffer PC, Bierregaard RO Jr (1995) Effects of forest fragmentation on understory hummingbirds in Amazonian Brazil. Conserv Biol 9:1085–1094
Townsend P, Levey D (2005) An experimental test of whether habitat corridors affect pollen transfer. Ecology 86:466–475
Uezu A, Metzger JP, Viellard JME (2005) Effects of structural and functional connectivity and patch size on the abundance of seven Atlantic Forest bird species. Biol Conserv 123:507–519
Vamosi JC, Knight TM, Steets JA, Mazer S, Burd M, Ashman T-L (2006) Pollination decays in biodiversity hotspots. Proc Natl Acad Sci U S A 103:956–961
Vergeer P, Rengelink R, Copal A, Ouborg NJ (2003) The interacting effects of genetic variation, habitat quality and population size on performance of Succisa pratensis. J Ecol 91:18–26
Wagenius S, Lonsdorf E, Neuhauser C (2007) Patch aging and the S-Allee effect: breeding system effects on the demographic response of plants to habitat fragmentation. Am Nat 169:383–397
Ward M, Johnson SD (2005) Pollen limitation and demographic structure in small fragmented populations of Brunsvigia radulosa (Amaryllidaceae). Oikos 108:253–262
Waser NM, Chittka L, Price MV, Williams NM, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Washitani I, Okayama Y, Sato K (1996) Spatial variation in female fertility related to interactions with flower consumers and pathogens in a forest metapopulation of Primula sieboldii. Res Popul Ecol 38:249–256
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–277
Winfree R, Griswold T, Kremen C (2007) Effect of human disturbance on bee communities in a forested ecosystem. Conserv Biol 21:213–223
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Acknowledgments
We are grateful to several people who contributed to this study. M.A. Aizen provided valuable support in our first steps in the course of fragmentation studies. We would also like to thank our lab mates, M.R. Darrigo and S. Del Carlo, as well as M. Perine, P.C. Fernandes and P.G.M. Cesário, who worked very hard with us during data collection in the field or in the laboratory; E.L. Catharino and G.A.D.C. Franco for P. suterella identification; C. Penz and K.S. Brown Jr. for butterfly identification, and G.A.R. Melo and I.A. Santos for bee identification. In addition, we thank J. R Nali from SABESP and the owners of forest fragments for permission to develop this study; N.L. Menezes and V. Angyalossy (Department of Botany, IB–USP) for logistic support in the plant anatomy laboratory; J.P. Metzger, P. Feinsinger and S. Del Carlo for their review of the manuscript; J.P. Metzger for giving us the opportunity to work on the project “Biodiversity conservation in fragmented landscapes in the Atlantic Plateau of São Paulo”; M.C. Ribeiro for his help with maps and GIS, and C.C. Ferraz, R. Khorsand and B.V. Young for their kind and dedicated help with the English language. We also want to thank Judith Bronstein and two anonymous sources who provided suggestions to improve this manuscript. Financial support was provided by FAPESP 99/12704-3 and 99/05123-4. All experiments complied with the current Brazilian laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judith Bronstein.
Rights and permissions
About this article
Cite this article
Lopes, L.E., Buzato, S. Variation in pollinator assemblages in a fragmented landscape and its effects on reproductive stages of a self-incompatible treelet, Psychotria suterella (Rubiaceae). Oecologia 154, 305–314 (2007). https://doi.org/10.1007/s00442-007-0830-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0830-z