Abstract
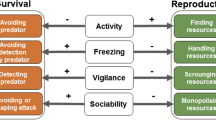
Metamorphosis has intrigued biologists for a long time as an extreme form of complex life cycles that are ubiquitous in animals. While investigated from a variety of perspectives, the ecological focus has been on identifying and understanding the ecological factors that affect an individual’s decision on when, and at what size, to metamorphose. Predation is a major factor that affects metamorphic decisions and a recent review by Benard (Annu Rev Ecol Evol Syst 35:651–673, 2004)) documented how predator cues induce metamorphic changes relative to model predictions. Importantly, however, real predators affect larval prey via several mechanisms beyond simple induction. In this paper, I contrast the leading models of metamorphosis, provide an overview of the multiple ways that predators can directly and indirectly affect larval growth and development (via induction, thinning, and selection), and identify how each process should affect the time to and size at metamorphosis. With this mechanistic foundation established, I then turn to the well-studied model system of larval amphibians to synthesize studies on: (1) caged predators (which cause only induction), and (2) lethal predators (which cause induction, thinning, and selection). Among the caged-predator studies, the chemical cues emitted by predators rarely induce a smaller size at metamorphosis or a shorter time to metamorphosis, which is in direct contrast to theoretical predictions but in agreement with Benard’s (Annu Rev Ecol Evol Syst 35:651–673, 2004) review based on a considerably smaller dataset. Among the lethal-predator studies, there is a diversity of outcomes depending upon the relative importance of induction versus thinning with the relative importance of the two processes appearing to change with larval density. Finally, I review the persistent effects of larval predators after metamorphosis including both phenotypic and fitness effects. At the end, I outline a number of future directions to allow researchers to continue gaining insight into how predators affect the metamorphic decisions of their prey.
Similar content being viewed by others
References
Abrams PA, Rowe L (1996) The effects of predation on the age and size of maturity of prey. Evolution 50:1052–1061
Adolph EF (1931) The size of body and size of environment in growth of tadpoles. Biol Bull 61:350–375
Altwegg R (2002a) Predator-induced life-history plasticity under time constraints in pool frogs. Ecology 83:2542–2551
Altwegg R (2002b) Trait-mediated indirect effects and complex life-cycles in two European frogs. Evol Ecol Res 4:519–536
Altwegg R, Reyer H-U (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Babbit KJ (2001) Behaviour and growth of southern leopard frog (Rana sphenocephala) tadpoles: effects of food and predation risk. Can J Zool 79:809–814
Babbit KJ, Tanner GW (1998) Effects of cover and predator size on survival and development of Rana utricularia tadpoles. Oecologia 114:258–262
Barnett HK, Richardson JS (2002) Predation risk and competition effects on the life-history characteristics of larval Oregon spotted frog and larval red-legged frog. Oecologia 132:436–444
Beachy CK (1997) Effect of predatory larval Desmognathus quadramaculatus on growth, survival, and metamorphosis of larval Eurycea wilderae. Copeia 1997:131–137
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life cycles. Annu Rev Ecol Evol Syst 35:651–673
Benard MF, Fordyce JA (2003) Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84:68–78
Berven KA (1981) Mate choice in wood frog, Rana sylvatica. Evolution 35:707–722
Berven KA (1982) The genetic basis of altitudinal variation in wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 36:962–983
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Bouskila A, Robinson ME, Roitberg BD, Tenhumberg B (1998) Life-history decisions under predation risk: Importance of a game perspective. Evol Ecol 12:701–715
Bragg AN (1956) Dimorphism and cannibalism in tadpoles of Scaphiopus bombifrons (Amphibia, Salientia). Southwest Nat 1:105–108
Brockelman WY (1969) An analysis of density effects and predation in Bufo americanus tadpoles. Ecology 50:632–644
Calef GW (1973) Natural mortality of tadpoles in a population of Rana aurora. Ecology 54:741–758
Chivers DP, Kiesecker JM, Marco A, Wildy EL, Blaustein AR (1999) Shifts in life history as a response to predation in western toads (Bufo boreas). J Chem Ecol 25:2455–2463
Day T, Rowe L (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat 159:338–350
Denver RJ, Mirhadi N, Phillips M (1998) Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 19:1859–1872
DeVito J, Chivers DP, Kiesecker JM, Belden LK, Blaustein AR (1999) Effects of snake predation on aggregation and metamorphosis of Pacific treefrog (Hyla regilla) larvae. J Herpetol 33:504–507
Emerson SB (1986) Heterochrony and frogs: the relationship of a life history trait to morphological form. Am Nat 127:167–183
Emerson SB, Travis J, Blouin M (1988) Evaluating a hypothesis about heterochrony: larval life-history traits and juvenile hind-limb morphology in Hyla crucifer. Evolution 42:68–78
Figiel CRJ, Semlitsch RD (1990) Population variation in survival and metamorphosis of larval salamanders (Ambystoma) in presence and absence of fish predation. Copeia 1990:818–826
Gerhardt HC (1994) The evolution of vocalization in frogs and toads. Annu Rev Ecol Syst 25:293–324
Gilbert LI, Frieden E (eds) (1981) Metamorphosis: a problem in developmental biology, 2nd edn. Plenum, New York
Gilliam JF (1982) Habitat use and competitive bottlenecks in size-structured fish populations. PhD dissertation. Michigan State University, East Lansing, Mich.
Hall BK, Wake MH (1999) The origin and evolution of larval forms. Academic Press, San Diego, Calif.
Herreid CF. Kinney S (1966) Survival of the Alaskan wood frog (Rana sylvatica) larvae. Ecology 47:1039–1041
Hamer AJ, Lane SJ, Mahony MJ (2002) The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia. Oecologia 132:445–452
Howard RD (1980) Mating behaviour and mating success in wood frogs, Rana sylvatica. Anim Behav 28:705–716
Kaltenbach JC (1996) Endocrinology of amphibian metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG (eds) Metamorphosis: postembryonic reprogramming of gene expression in amphibian and insect cells. Academic Press, San Diego, Calif., pp 403–431
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kiesecker JM, Blaustein AR (1998) Effects of introduced bullfrogs and smallmouth bass on microhabitat use, growth, and survival of native red-legged frogs (Rana aurora). Conserv Biol 12:776–787
Kiesecker JM, Chivers DP, Anderson M, Blaustein AR (2002) Effect of predator diet on life history shifts of red-legged frogs, Rana aurora. J Chem Ecol 28:1007–1015
Kraft PG, Wilson RS, Franklin CE (2005) Predator-mediated phenotypic plasticity in tadpoles of the striped marsh frog, Limnodynastes peronii. Aust Ecol 30:558–663
Kurzava LM, Morin PJ (1998) Tests of functional equivalence: complementary roles of salamanders and fish in community organization. Ecology 79:477–489
Lane SJ, Mahony MJ (2002) Larval anurans with synchronous and asynchronous developmental periods: contrasting responses to water reduction and predator presence. J Anim Ecol 71:780–792
Lardner B (2000) Morphological and life history responses to predators in larvae of seven anurans. Oikos 88:169–180
Laurila A, Järvi-Laturi M, Pakkasmaa S, Merila J (2004) Temporal variation in predation risk: stage dependency, graded responses and fitness costs in tadpole antipredator defense. Oikos 107:90–99
Laurila A, Kujasalo J (1999) Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) tadpoles. J Anim Ecol 68:1123–1132
Laurila A, Kujasalo J, Ranta E (1998) Predator-induced changes in life-history in two anuran tadpoles: effects of predator diet. Oikos 83:307–317
Law R (1979) Optimal life histories under age-specific predation. Am Nat 114:399–417
Leips J, Travis J (1994) Metamorphic responses to changing food levels in two species of hylid frogs. Ecology 75:1345–1356
Loman J (2002) Temperature, genetic and hydroperiod effects on metamorphosis of brown frogs Rana arvalis and R. temporaria in the field. J Zool 258:115–129
Ludwig D, Rowe L (1990) Life history strategies for energy gain and predator avoidance under time constraints. Am Nat 135:686–707
Michod RE (1979) Evolution of life histories in response to age-specific mortality factors. Am Nat 113:531–550
Mills NE, Semlitsch RD (2004) Competition and predation mediate the indirect effects of an insecticide on southern leopard frogs. Ecol Appl 14:1041–1054
Morin PJ (1983) Predation, competition, and composition of larval anuran guilds. Ecol Monogr 53:119–138
Morin PJ (1986) Interactions between intraspecific competition and predation in an amphibian predator–prey system. Ecology 67:713–720
Morin PJ (1987) Predation, breeding asynchrony, and outcome of competition among treefrog tadpoles. Ecology 68:675–683
Morin PJ (1995) Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology 76:133–149
Newman RA (1992) Adaptive plasticity in amphibian metamorphosis. Bioscience 42:671–678
Nicieza AG (2000) Interacting effects of predation risk and food availability on larval anuran behaviour and development. Oecologia 123:497–505
Nyström P, Svensson O, Lardner B, Brönmark C, Granéli W (2001) The influence of multiple introduced predators on a littoral pond community. Ecology 82:1025–1039
Orizaolo G, Braña F (2005) Plasticity in newt metamorphosis: the effect of predation at embryonic and larval stages. Freshwater Biol 50:438–446
Parris MJ, Beaudoin JG (2004) Chytridiomycosis impacts predator–prey interactions in larval amphibian communities. Oecologia 140:626–632
Peacor SD (2002) Positive effect of predators on prey growth rate through induced modifications of prey behaviour. Ecol Lett 5:77–85
Pearman PB (1995) Effects of pond size and consequent predator density on two species of tadpoles. Oecologia 102:1–8
Pechenik JA, Wendt DE, Jarrett JN (1998) Metamorphosis is not a new beginning. Bioscience 48:901–910
Petranka JW, Kats LB, Sih A (1987) Predator–prey interactions among fish and larval amphibians: Use of chemical cues to detect predatory fish. Anim Behav 35:420–425
Relyea RA (2000) Trait-mediated effects in larval anurans: reversing competition with the threat of predation. Ecology 81:2278–2289
Relyea RA (2001a) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2001b) The lasting effects of adaptive plasticity: Predator-induced tadpoles become long-legged frogs. Ecology 82:1947–1955
Relyea RA (2001c) The relationship between predation risk and anti-predator responses in larval anurans. Ecology 82:541–554
Relyea RA (2002a) Competitor-induced plasticity in tadpoles: consequences, cues, and connections to predator-induced plasticity. Ecol Monogr 72:523–540
Relyea RA (2002b) Costs of phenotypic plasticity. Am Nat 159:272–282
Relyea RA (2002c) Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr 72:77–93
Relyea RA (2002d) The many faces of predation: how selection, induction, and thinning combine to alter prey phenotypes. Ecology 83:1953–1964
Relyea RA (2003a) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Relyea RA (2003b) Predator cues and pesticides: a double dose of danger for amphibians. Ecol Appl 13:1515–1521
Relyea RA (2003c) Predators come and predators go: the reversibility of predator-induced traits. Ecology 84:1840–1848
Relyea RA (2004) Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology 85:172–179
Relyea RA, Auld JR (2004) Having the guts to compete: how intestinal plasticity explains costs of inducible defenses. Ecol Lett 7:869–875
Relyea RA, Auld JR (2005) Predator- and competitor-induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology 86:1723–1729
Relyea RA, Hoverman JT (2003) The impact of larval predators and competitors on the morphology and fitness of juvenile tree frogs. Oecologia 134:596–604
Relyea RA, Werner EE (1999) Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology 80:2117–2124
Relyea RA, Werner EE (2000) Morphological plasticity of four larval anurans distributed along an environmental gradient. Copeia 2000:178–190
Resetarits WJ Jr, Rieger JF, Binkley CA (2004) Threat of predation negates density effects in larval gray treefrogs. Oecologia 138:532–538
Rose CS (2005) Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol Evol 20:129–135
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72:413–427
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer, Sunderland, Mass.
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defense: the roles of alarm cues and kairomones for inducing prey defenses. Ecol Lett 8:505–512
Semlitsch RD (1993) Effects of different predators on survival and development of tadpoles from hybridogenetic Rana esculenta complex. Oikos 67:40–46
Semlitsch RD, Scott DC, Pechmann JHK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Skelly DK (1992) Field evidence for a cost of behavioral antipredator response in a larval amphibian. Ecology 73:704–708
Skelly DK (1994) Activity level and susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Smith DC (1983) Factors controlling tadpole populations of chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Smith DC, Van Buskirk J (1995) Phenotypic design, plasticity, and ecological performance in two tadpole species. Am Nat 145:211–233
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585
Sredl MJ, Collins JP (1992) The interaction of predation, competition, and habitat complexity in structuring an amphibian community. Copeia 1992:607–614
Stearns S, Koella JC (1986) The evolution of phenotypic plasticity in life history traits: predictions of reaction norms for age and size of maturity. Evolution 40:893–914
Tollrian R, Harvell D (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, N.J
Travis J, Keen WH, Julianna J (1985) The role of relative body size in a predator–prey relationship between dragonfly naiads and larval anurans. Oikos 45:59–65
Truman JW, Riddiford LM (2002) Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol 47:467–500
Turner FB (1962) The demography of frogs and toads. Q Rev Biol 37:303–314
Van Buskirk J (1988) Interactive effects of dragonfly predation in experimental pond communities. Ecology 69:857–867
Van Buskirk J (2000) The costs of an inducible defense in anuran larvae. Ecology 81:2813–2821
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160:87–102
Van Buskirk J, McCollum SA, Werner EE (1997) Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51:1983–1992
Van Buskirk J, Relyea RA (1998) Natural selection for phenotypic plasticity: predator-induced morphological responses in tadpoles. Biol J Linn Soc 65:301–328
Van Buskirk J, Saxer G (2001) Delayed costs of an induced defense in tadpoles? Morphology, hopping, and development rate at metamorphosis. Evolution 55:821–829
Van Buskirk J, Schmidt BR (2000) Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecology 81:3009–3028
Van Buskirk J, Yurewicz KL (1998) Effects of predators on prey growth rate: relative contributions of thinning and reduced activity. Oikos 82:20–28
Vonesh JR (2005) Sequential predator effects across three life stages of the African tree frog, Hyperolius spinigularis. Oecologia 143:280–290
Vonesh JR, Bolker BM (2005) Compensatory larval responses shift trade-offs associated with predator-induced hatching plasticity. Ecology 86:1580–1591
Vonesh JR, Warkentin KM (2006) Opposite shifts in size at metamorphosis in response to larval and metamorph predators. Ecology 87:556–562
Vorndran IC, Reichwaldt E, Nürnberger B (2002) Does differential susceptibility to predation in tadpoles stabilize the Bombina hybrid zone? Ecology 83:1648–1659
Wassersug RJ (1997) Where tadpole meets world—observations and speculations on biomechanical and biochemical factors that influence metamorphosis in anurans. Am Zool 37:124–136
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and optimal size at transformation. Am Nat 128:319–341
Werner EE (1988) Size, scaling, and evolution of complex life cycles. In: Ebenman B, Persson L (eds) Size-structured populations. Springer, Berlin Heidelberg New York, pp 60–81
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Werner EE, McPeek MA (1994) Direct and indirect effects of predators on two anuran species along an environmental gradient. Ecology 75:1368–1382
Wilbur HM (1972) Competition, predation, and structure of Ambystoma–Rana sylvatica community. Ecology 53:3–21
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Wilbur HM, Fauth JE (1990) Experimental aquatic food webs: interactions between two predators and two prey. Am Nat 135:176–204
Wildy EL, Chivers DP, Blaustein AR (1999) Shifts in life-history traits as a response to cannibalism in larval long-toed salamanders (Ambystoma macrodactylum). J Chem Ecol 25:2337–2346
Wilbur HM, Morin PJ, Harris RN (1983) Salamander predation and structure of experimental communities: anuran responses. Ecology 64:1423–1429
Zug GR (1972) Anuran locomotion: structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia 1972:613–624
Acknowledgements
This article was inspired by the 2003 Joint Meeting of Ichthyologists and Herpetologists in which I presented these ideas as part of a symposium celebrating the 30th anniversary of the Wilbur and Collins’ (1973) model of metamorphosis. Like many ecologists, I would like to express my appreciation to Henry Wilbur and Jim Collins for their inspirational work. Josh Auld, Mike Benard, Jason Hoverman, Steve Kohler, Nancy Schoeppner, and Miguel Tejedo provided very helpful insights on the manuscript. I thank the National Science Foundation for its continued support of my research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Relyea, R.A. Getting out alive: how predators affect the decision to metamorphose. Oecologia 152, 389–400 (2007). https://doi.org/10.1007/s00442-007-0675-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0675-5