Abstract
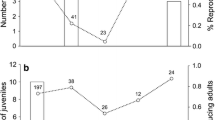
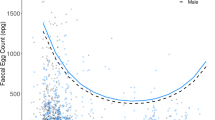
Host age is one of the key factors in host–parasite relationships as it possibly affects infestation levels, parasite-induced mortality of a host, and parasite distribution among host individuals. We tested two alternative hypotheses about infestation pattern and survival under parasitism in relation to host age. The first hypothesis assumes that parasites are recruited faster than they die and, thus, suggests that adult hosts will show higher infestation levels than juveniles because the former have more time to accumulate parasites. The second hypothesis assumes that parasites die faster than they are recruited and, thus, suggests that adults will show lower infestation levels because of acquired immune response and/or the mortality of heavily infested juveniles and, thus, selection for less infested adults. As the negative effects of parasites on host are often intensity-dependent, we expected that the age-related differences in infestation may be translated to lower or higher survival under parasitism of adults, in the cases of the first and the second hypotheses, respectively. We manipulated ectoparasite numbers using insecticide and assessed the infestation pattern in adult and juvenile gerbils (Gerbillus andersoni) in the Negev Desert. We found only a partial support for age-dependent parasitism. No age-related differences in infestation and distribution among host individuals were found after adjusting the ectoparasite numbers to the host’s surface area. However, age-related differences in survival under parasitism were revealed. The survival probability of parasitized juveniles decreased in about 48% compared to unparasitized hosts while the survival probability of adults was not affected by ectoparasites. Our results suggest that the effect of host age on host–parasite dynamics may not explicitly be determined by age-dependent differences in ectoparasite recruitment or mortality processes but may also be affected by other host-related and parasite-related traits.




Similar content being viewed by others
References
Abramsky Z (1984) Population biology of Gerbillus allenbyi in Northern Israel. Mammalia 48:197–206
Alberts JR, Cramer CP (1988) Ecology and experience: sources of means and meaning of developmental change. In: Blass EM (ed) Developmental psychobiology and behavioral ecology, vol 9. Plenum, New York, pp 1–62
Anderson RM, Gordon DM (1982) Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology 85:373–398
Anderson RM, May RM (1978) Regulation and stability of host–parasite population interactions. I. Regulatory processes. J Anim Ecol 47:219–247
Atkinson CT, Van Riper III C (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird–parasite interactions: ecology, evolution, and behaviour, vol 2. Oxford University Press, Oxford, pp 19–48
Boulinier T, McCoy KD, Sorci G (2001) Dispersal and parasitism. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal, vol 12b. Oxford University Press, Oxford, pp 169–179
Brinck-Lindroth G (1968) Host spectra and distribution of fleas of small mammals in Swedish Lapland. Opusc Entomol 33:327–358
Byrom AE, Krebs CJ (1999) Natal dispersal of juvenile arctic ground squirrels in the boreal forest. Can J Zool 77:1048–1059
Cox DR (1972) Regression models and life tables. J R Stat Soc Lond Ser B 26:103–110
Davies CR, Gavgani ASM (1999) Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology 119:247–257
Degen AA, Kam M (1991) Average daily metabolic-rate of gerbils of 2 species—Gerbillus pyramidum and Gerbillus allenbyi. J Zool 223:143–149
Fichet-Calvet E, Wang JF, Jomaa I, Ismail RB, Ashford RW (2003) Patterns of the tapeworm Raillietina trapezoides infection in the fat sand rat Psammomys obesus in Tunisia: season, climatic conditions, host age and crowding effects. Parasitology 126:481–492
Fuller CA, Blaustein AR (1996) Effects of the parasite Eimeria arizonensis on survival of deer mice (Peromyscus maniculatus). Ecology 77:2196–2202
Goater CP, Semlitsch RD, Bernasconi MV (1993) Effects of body size and parasite infection on the locomotory performance of juvenile toads, Bufo bufo. Oikos 66:129–136
Gregory RD, Montgomery SSJ, Montgomery WI (1992) Population biology of Heligmosomoides polygyrus (Nematoda) in the Wood Mouse. J Anim Ecol 61:749–757
Grenfell BT, Dobson AP (1995) Ecology of infectious diseases in natural populations. Cambridge University Press, Cambridge
Gurarie D, King CH (2005) Heterogeneous model of schistosomiasis transmission and long-term control: the combined influence of spatial variation and age-dependent factors on optimal allocation of drug therapy. Parasitology 130:49–65
Harrison DL, Bates PJJ (1991) The mammals of Arabia, 2nd edn. Harrison Zoological Museum, Sevenoaks
Hawlena H, Abramsky Z, Krasnov BR (2005) Age-biased parasitism and density-dependent distribution of fleas (Siphonaptera) on a desert rodent. Oecologia 146:200–208
Henricson J (1977) The abundance and distribution of Diphyllobothrium dentriticum (Nitzsch) and D. ditremum (Creplin) in the char Salvelinus alpinus. J Fish Biol 11:231–248
Heusner AA (1985) Body size and energy-metabolism. Annu Rev Nutr 5:267–295
Hudson PJ, Dobson AP (1995) Macroparasites: observed pattern in naturally fluctuating animal populations. In: Grenfell BT, Dobson AP (eds) Ecology of infectious diseases in natural populations, vol 5. Cambridge University Press, Cambridge, pp 144–176
Hudson PJ, Dobson AP (1997) Host–parasite processes and demographic consequences. In: Clayton DH, Moore J (eds) Host–parasite evolution. General principles and avian models, vol 7. Oxford University Press, New York, pp 128–154
Khokhlova IS, Spinu M, Krasnov BR, Degen AA (2004) Immune response to fleas in a wild desert rodent: effect of parasite species, parasite burden, sex of host and host parasitological experience. J Exp Biol 207:2725–2733
Knudsen R, Amundsen PA, Klemetsen A (2002) Parasite-induced host mortality: indirect evidence from a long-term study. Environ Biol Fishes 64:257–265
Kuperman BI, Matey VE, Barlow SB (2002) Flagellate Cryptobia branchialis (Bodonida: Kinetoplastida), ectoparasite of tilapia from the Salton Sea. Hydrobiologia 473:93–102
Lehmann T (1992) Ectoparasite impacts on Gerbillus andersoni allenbyi under natural conditions. Parasitology 104:479–488
Lehmann T (1993) Ectoparasites—direct impact on host fitness. Parasitol Today 9:8–13
Marshall AG (1981) The ecology of ectoparasitic insects. Academic Press, London
Martin TE, Moller AP, Merino S, Clobert J (2001) Does clutch size evolve in response to parasites and immunocompetence? Proc Natl Acad Sci USA 98:2071–2076
Moller AP, Allander K, Dufva R (1990) Fitness effects of parasites on passerine birds: a review. In: Blondel J, Gosler A, Lebreton JD, McCleery RH (eds) Population biology of passerine birds: an integrated approach. Springer, Berlin Heidelberg New York, pp 269–280
Mooring MS, Blumstein DT, Stoner CJ (2004) The evolution of parasite-defence grooming in ungulates. Biol J Linn Soc 81:17–37
Munn AJ, Dawson TJ (2003) Energy requirements of the red kangaroo (Macropus rufus): impacts of age, growth and body size in a large desert-dwelling herbivore. J Comp Physiol B Biochem Syst Environ Physiol 173:575–582
Murray DL, Cary JR, Keith LB (1997) Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. J Anim Ecol 66:250–264
Neuhaus P (2003) Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus). Proc R Soc Lond B 270:S213–S215
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Puchala P (2004) Detrimental effects of larval blow files (Protocalliphora azurea) on nestlings and breeding success of Tree Sparrows (Passer montanus). Can J Zool 82:1285–1290
Quinnell RJ (1992) The population-dynamics of Heligmosomoides polygyrus in an enclosure population of wood mice. J Anim Ecol 61:669–679
Reinert HK (1993) Habitat selection in snakes. In: Seigel RA, Collins JT (eds) Snakes. Ecology and behavior. McGraw-Hill, New York, pp 209–211
Richner H (1998) Host–parasite interactions and life-history evolution. Zool Anal Complex Syst 101:333–344
Rosenzweig ML, Abramsky Z (1997) Two gerbils of the Negev: a long-term investigation of optimal habitat selection and its consequences. Evol Ecol 11:733–756
Rousset F, Thomas F, deMeeus T, Renaud F (1996) Inference of parasite-induced host mortality from distributions of parasite loads. Ecology 77:2203–2211
Schall JJ, Bennett AF, Putnam RW (1982) Lizards infected with malaria: physiological and behavioral consequences. Science 217:1057–1059
Schoenig GP (1995) Mammalian toxicology of Pyrethrum extracts. In: Casida JE, Quistad GB (eds) Pyrethrum flowers production, chemistry, toxicology, and uses, vol 12. Oxford University Press, New York, pp 249–257
Smit FGAM (1962) Siphonaptera collected from moles and their nests at Wilp, Netherlands, by Jhr. W.C. van Heurn. Tijdschr Entomol 105:29–44
Sol D, Jovani R, Torres J (2003) Parasite mediated mortality and host immune response explain age-related differences in blood parasitism in birds. Oecologia 135:542–547
Sonenshine DE (1985) Pheromones and other semiochemicals of the Acari. Annu Rev Entomol 30:1–28
Sorci G (1996) Patterns of haemogregarine load, aggregation and prevalence as a function of host age in the lizard Lacerta vivipara. J Parasitol 82:676–678
Southwood TRE (1978) Ecological methods, with particular reference to the study of insect populations. Wiley, New York
Stenseth NC, Lidicker WZ (1992) Animal dispersal: small mammals a model. Chapman and Hall, New York
Theodor O, Costa M (1967) A survey of the parasites of wild mammals and birds in Israel. Part one: ectoparasites. Goldberg, Jerusalem
Ulmanen I, Myllymaki A (1971) Species composition and numbers of fleas (Siphonaptera) in a local population of the field vole, Microtus agrestis (L.). Ann Zool Fenn 8:374–384
Van Vuren D (1996) Ectoparasites, fitness, and social behaviour of yellow-bellied marmots. Ethology 102:686–694
Wikel SK (1996) Immunology of the skin. In: Wikel SK (ed) The immunology of host–ectoparasitic arthropod relationships, vol 1. CABI, Wallingford, UK, pp 1–29
Acknowledgements
Dror Hawlena, Ofer Ovadia, Moshe Kiflawi and Amos Bouskila were involved in interesting discussions. We thank Nadezhda Burdelova, Maria Stanukovich and Dmitry Apanaskevich for their help in ectoparasite identification. We are grateful to Dudi Greenbaum and Arnon Tsairi for their invaluable help with all aspects of the study. We also appreciate the thoughtful comments of Burt Kotler, Gideon Wasserberg, Dirk Van Vuren, and F. Stephen Dobson on an earlier version of the manuscript. Financial support during this study was provided by Ministry of Science, Culture and Sport of Israel (grant 01-18-00331). This is publication no.196 of the Ramon Science Center and no. 503 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hannu Ylonen
Rights and permissions
About this article
Cite this article
Hawlena, H., Abramsky, Z. & Krasnov, B.R. Ectoparasites and age-dependent survival in a desert rodent. Oecologia 148, 30–39 (2006). https://doi.org/10.1007/s00442-005-0345-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0345-4