Abstract
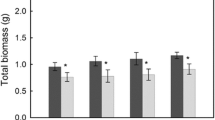
The effect of the community composition of soil microbes on ecosystem processes has received relatively little attention. Here we examined the variation in soil microbial composition in a Yellowstone National Park grassland and the effect of that variation on the growth, in a greenhouse, of the dominant grass in the community. Plants and their rhizospheric soil were collected from paired, Poa pratensis-dominated grassland plots located inside and outside a 40-year-old exclosure. P. pratensis aboveground, belowground, and whole plant growth were greater in pots with soil communities from grazed grassland compared to fenced grassland, indicating (1) soil microbial communities differed, and (2) this difference influenced the growth of the plant that dominated both grasslands. Treating pots with fungicide (benomyl) suppressed the soil community influence, indicating that different fungal communities caused the soil microbe effect. In addition, two lines of evidence are consistent with the hypothesis that arbuscular mycorrhizal fungal (AMF) species composition affected P. pratensis: (1) a divergence in AMF spore communities in the two field soils, and (2) little evidence of pathogenic fungi. These findings emphasize the need to examine the role that the composition of the soil microbial community plays in controlling terrestrial ecosystems.

Similar content being viewed by others
References
Allen MF, Richards GH, Busso CA (1989) Influence of clipping and soil water status on vesicular-arbuscular mycorrhizae of two semi-arid tussock grasses. Biol Fertil Soil 8:285–289
Atlas R M, Bartha R (1993) Microbial ecology: fundamentals and applications. Benjamin Cummings, Redwood City, Calif.
Augspurger CK (1984) Seedling survival of tropical tree species: interactions of dispersal distance, light gaps, and pathogens. Ecology 65:1705–1712
Bever JD (1994) Feedback between plants and their soil communities in an old field. Ecology 75:1965–1977
Bever JD (2002) Host-specificity of AM fungal population growth rates can generate feedback on plant growth. Plant Soil 244:281–290
Bianchi S, Jones CG, Shachak M (1989) Positive feedback of consumer population density on resource supply. Trends Ecol Evol 4:234–238
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. ACIAR Monogr 32, Pirie, Canberra
Degens BP (1999) Catabolic response profiles differ between microorganisms grown in soils. Soil Biol Biochem 31:475–477
Eom A-H, Wilson GWT, Hartnett DC (2001) Effects of ungulate grazers on arbuscular mycorrhizal symbiosis and fungal community structure in tallgrass prairie. Mycologia 93:233–242
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69:57–68
Fester T, Maier W, Strack D (1999) Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 8:241–246
Fitter AH (1977) Influence of mycorrhizal infection on competition for phosphorus and potassium by two grasses. New Phytol 79:119–125
Fitter AH, Nichols R (1988) The use of benomyl to control infection by vesicular-arbuscular mycorrhizal fungi. New Phytol 110:201–206
Frank DA, Groffman PM (1998) Ungulate vs. landscape control of soil C and N processes in grasslands of Yellowstone National Park. Ecology 79:2229–2241
Frank DA, McNaughton SJ (1992) The ecology of plants, large mammalian herbivores, and drought in Yellowstone National Park. Ecology 73:2043–2058
Frank DA, McNaughton SJ, Tracy BF (1998) The ecology of the earth’s grazing ecosystems. BioScience 48:513–521
Frank DA, Kuns MM, Guido DR (2002) Consumer control of grassland plant production. Ecology 83:602–606
Gehring CA, Whitham TG (1994) Interactions between aboveground herbivores and the mycorrhizal mutualists of plants. Trends Ecol Evol 9:251–255
Gehring CA, Whitham TG (2002) Mycorrhizae-herbivore interactions: population and community consequences. In: van der Heijden MGA, Sanders I (eds) Mycorrhizal ecology. Springer, Berlin Heidelberg New York, pp 295–320
Gehring CA, Wolf JE, Theimer TC (2002) Terrestrial vertebrates promote arbuscular mycorrhizal fungal diversity and inoculum potential in a rainforest soil. Ecol Lett 5:540–548
Halverson LJ, Jones TM, Firestone MK (2000) Release of intracellular solutes by four soil bacteria exposed to dilution stress. Soil Sci Soc Am J 64:1630–1637
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Hartnett DC, Hetrick BAD, Wilson GWT, Gibson DJ (1993) Mycorrhizal influence on intra- and inter-specific neighbor interactions among co-ocurring prairie grasses. J Ecol 81:787–795
Hector A, Scmid B, Beierkuhnlein C, Caldeira M C, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller PS, Good J, Harris R, Högberg P, Huss-Danell K, Joshi J, Jumpponen A, Körner C, Leadley PW, Loreau M, Minns A, Mulder CPH, O’Donovan G, Otway SJ, Pereira JS, Prinz A, Read DJ, Scherer-Lorenzen M, Schulze E-D, Siamantziouras A-SD, Spehn E M, Terry AC, Troumbis AY, Woodward F I, Yachi S, Lawton JH (1999) Plant diversity and productivity experiments in European grasslands. Science 286:1123–1127
Heijden MGA van der, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
Hetrick BAD, Wilson GWT, Owensby CE (1990) Mycorrhizal influences on big bluestem rhizome regrowth and clipping tolerance. J Range Manage 43:286–290
Holah JC, Alexander HM (1999) Soil pathogenic fungi have the potential to affect the co-existence of two tallgrass prairie species. J Ecol 87:598–608
Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277:1302–1305
Houston DB (1982) The northern Yellowstone elk: ecology and management. Macmillan, New York
Huntly N (1991) Herbivores and the dynamics of communities and ecosystems. Annu Rev Ecol Syst 22:477–503
Huston MA (1997) Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110:449–460
Johnson NC, O’Dell TE, Bledsoe CS (1999) Methods for ecological studies of mycorrhizae. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York
Jones TH, Bradford MA (2001) Assessing the functional implication of soil biodiversity in ecosystems. Ecol Res 16:845–858
Kiers ET, Lovelock CE, Krueger EK, Herre EA (2000) Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: implications for tropical forest diversity. Ecol Lett 3:106–113
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Lieth H, Whittaker RH (1975) Primary productivity of the biosphere. Ecological studies 14. Springer, Berlin Heidelberg New York
Madigan MT, Martinko JM, Parker J (2000) Biology of microorganisms, 9th edn. Prentice-Hall, Upper Saddle River, N.J.
Mazancourt C de, Loreau M, Abbadie L (1999) Grazing optimization and nutrient cycling: potential impact of large herbivores in a savanna system. Ecol Appl 9:784–797
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
McNaughton SJ (1976) Serengeti migratory wildebeest: facilitation of energy flow by grazing. Science 191:92–94
Meagher MM (1973) The bison of Yellowstone National Park. NPS Sci Monogr Ser 1. United States Department of Interior, Washington, D.C.
Meentemeyer V (1978) Macroclimate and lignin control of decomposition rates. Ecology 59:465–472
Minchin PR (1999) DECODA: Database for ecological data. ANUTECH, Canberra, Australia
Naeem A, Hahn DR, Schuurman G (2000) Producer-decomposer co-dependency influences biodiversity effects. Nature 403:762–764
Nieto-Sotelo J, Ho T-HD (1987) Absence of heat shock protein synthesis in isolated mitochodria and plastids from maize. J Biol Chem 262:12288–12292
Packer A, Clay K (2000) Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404:278–281
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic, San Diego, Calif.
Putten WH van der, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53–56
Ratti N, Kumar S, Verma HN, Gautam SP (2001) Improvement in bioavailability of tricalcium phosphate to Cymbopogon martinii var. motia by rhizobacteria, AMF, and Azospirillum inoculation. Microbiol Res 156:145–149
Ronsheim ML, Andersen SE (2001) Population-level specificity in the plant-mycorrhizae association alters intraspecific interactions among neighboring plants. Oecologia 128:77–84
Rosenzweig ML (1968) Net primary productivity of terrestrial communities: predictions from climatological data. Am Nat 102:67–74
Schenck NC, Pérez Y (1990) Manual for the identification of VA mycorrhizal fungi, 3rd edn. Synergistic, Gainsville, Fla.
Schimel JP, Gulledge J (1998) Microbial community structure and global trace gases. Global Change Biol 4:745–758
Shearer G, Kohl DH, Virginia RH, Bryan BA, Skeeters JL, Nilsen ET, Sharifi MR, Rundel PW (1983) Estimates of N2-fixation from variation in the natural abundance of 15N in Sonoran desert ecosystems. Oecologia 56:365–373
Streitwolf-Engel R, Van der Heijden MGA, Weimken A, Sanders IR (2001) The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 82:2846–2859
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1847
Wallace LL (1981) Growth, morphology and gas exchange of mycorrhizal and nonmycorrhizal Panicum coloratum L., a C4 grass species, under different clipping and fertilization regimes. Oecologia 49:272–278
Wallace LL (1987) Effects of clipping and soil compaction on growth, morphology and mycorrhizal colonization of Schizachyrium scoparium, a C4 bunchgrass. Oecologia 72:423–428
Wolf JE, Johnson NC, Rowland DL, Reich PB (2003) Elevated CO2 and plant species richness impact arbuscular mycorrhizal fungal spore communities. New Phytol 157:579–588
Youngberg CT, Wollum AG (1976) Nitrogen accretion in developing Ceanothus velutinus stands. Soil Sci Soc Am Proc 40:109–112
Acknowledgements
We thank V. Kurth and H. Loring for collecting the plant and soil material, J. Wolf, G. Cuenca, and N.C. Johnson for assistance with the AMF spore analyses, and S.J. McNaughton for commenting on an early draft. Discussions with C. de Mazancourt and S. Hartley stimulated this study and NSF grants DEB-9726569 to D. Frank and DEB-0087017 to C. Gehring supported the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frank, D.A., Gehring, C.A., Machut, L. et al. Soil community composition and the regulation of grazed temperate grassland. Oecologia 137, 603–609 (2003). https://doi.org/10.1007/s00442-003-1385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1385-2