Abstract
Mesenchymal stem cells (MSCs) have shown great potential in treating autoimmune diseases due to their immunomodulatory capability, which has been verified in both animal experiments and clinical trials. Psoriasis is a chronic and remitting immune-related disease. Limited studies have demonstrated that MSCs might be an effective therapeutic approach for managing psoriasis, whose underlying mechanism remains to be elucidated. In our present study, human umbilical cord-derived MSCs (hUC-MSCs) were subcutaneously injected into mice with imiquimod (IMQ)-induced psoriasis-like skin inflammation to explore the feasibility of this cellular therapy. The severity of psoriasis-like dermatitis was evaluated by cumulative psoriasis area and severity index score and epidermal thickness of skin tissue sections. Flow cytometric analysis was utilized to detect T helper cells, regulatory T cells, and γδ T cells in skin-draining lymph nodes. Real-time quantitative polymerase chain reaction and enzyme-linked immunosorbent assay were used to assess the expression levels of psoriasis-related cytokines and chemokines in mouse dorsal skin lesions. We discovered that hUC-MSCs drastically diminished the severity of IMQ-induced psoriasis-like dermatitis and suppressed inflammatory cell response. Although the tail vein injection of hUC-MSCs was also effective, it was correlated with higher mortality owing to pulmonary embolism. By comparison, subcutaneous injection with two million hUC-MSCs was identified to be the optimal therapeutic strategy. Furthermore, we uncovered that hUC-MSCs might repress skin inflammation probably through inhibiting interleukin-17-producing γδ T cells. In conclusion, subcutaneous administration of hUC-MSCs might be a promising therapeutic approach for psoriasis. Our findings provide novel insights into the underpinning mechanism of hUC-MSC treatment in the management of psoriasis.
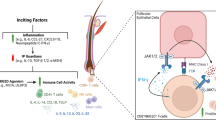
Graphical abstract








Similar content being viewed by others
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- hUC-MSCs:
-
Human umbilical cord-derived MSCs
- IMQ:
-
Imiquimod
- RA:
-
Rheumatoid arthritis
- SOP:
-
Standard operating procedure
- IL:
-
Interleukin
- BCA:
-
Bicinchoninic acid
- TNF:
-
Tumor necrosis factor
- IFN:
-
Interferon
- LN:
-
Lymph nodes
- BM:
-
Bone marrow
- PASI:
-
Psoriasis area severity index
- TGF:
-
Transforming growth factor
- Th17:
-
T helper 17
- PBS:
-
Phosphate-buffered saline
- H&E:
-
Hematoxylin and eosin
- SLE:
-
Systemic lupus erythematosus
- ECM1:
-
Extracellular matrix protein 1
- MCAM:
-
Melanoma cell adhesion molecule
References
Albanesi C, Madonna S, Gisondi P, Girolomoni G (2018) The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol 9:1549
Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J (2016) JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One 11:e0164080
Arturo J, Perez C, Larios L, Segura O, Bastidas Y, Segura O, Lucena C, Lucena E, Ruiz C (2014) Immunomodulation and induction of remission in crohn's disease with autologous expanded mesenchymal stem cells. case report. Cytotherapy 16:S101
Bandyopadhyay M, Larregina AT (2020) Keratinocyte-polyamines and dendritic cells: a bad duet for psoriasis. Immunity 53:16–18
Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13:392–402
Blauvelt A, Chiricozzi A (2018) The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 55:379–390
Boehncke WH, Schön MP (2015) Psoriasis. Lancet (london, England) 386:983–994
Chen H, Niu JW, Ning HM, Pan X, Li XB, Li Y, Wang DH, Hu LD, Sheng HX, Xu M et al (2016) Treatment of psoriasis with mesenchymal stem cells. Am J Med 129:e13-14
Chen M, Peng J, Xie Q, Xiao N, Su X, Mei H, Lu Y, Zhou J, Dai Y, Wang S et al (2019a) Mesenchymal stem cells alleviate moderate-to-severe psoriasis by reducing the production of type i interferon (IFN-I) by plasmacytoid dendritic cells (pDCs). Stem Cells Int 2019:6961052
Chen Y, Yu Q, Hu Y, Shi Y (2019b) Current research and use of mesenchymal stem cells in the therapy of autoimmune diseases. Curr Stem Cell Res Ther 14:579–582
Choi EW, Shin IS, Song JW, Lee M, Yun TW, Yang J, Choi KS, Kim SJ (2016) Effects of transplantation of CTLA4Ig-overexpressing adipose tissue-derived mesenchymal stem cells in mice with sustained severe rheumatoid arthritis. Cell Transplant 25:243–259
Conrad C, Gilliet M (2018) Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol 54:102–113
De Jesus MM, Santiago JS, Trinidad CV, See ME, Semon KR, Fernandez MO Jr, Chung FS (2016) Autologous adipose-derived mesenchymal stromal cells for the treatment of psoriasis vulgaris and psoriatic arthritis: a case report. Cell Transplant 25:2063–2069
Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, Dave M, Friton J, Nair A, Camilleri ET et al (2017) Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 153:59-62.e52
Fitzgerald O, Winchester R (2014) Editorial: emerging evidence for critical involvement of the interleukin-17 pathway in both psoriasis and psoriatic arthritis. Arthritis & Rheumatology (hoboken, NJ) 66:1077–1080
Gallo R, Gambelli F, Gava B, Sasdelli F, Tellone V, Masini M, Marchetti P, Dotta F, Sorrentino V (2007) Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death Differ 14:1860–1871
Gray EE, Suzuki K, Cyster JG (2011) Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol 186:6091–6095
Han Y, Mora J, Huard A, da Silva P, Wiechmann S, Putyrski M, Schuster C, Elwakeel E, Lang G, Scholz A et al (2019) IL-38 Ameliorates skin inflammation and limits IL-17 production from γδ T cells. Cell Rep 27:835-846.e835
Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B (2015) Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol 45:3022–3033
Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA (2013) CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol 133:973–979
Hou R, Liu R, Niu X, Chang W, Yan X, Wang C, Li J, An P, Li X, Yin G et al (2014a) Biological characteristics and gene expression pattern of bone marrow mesenchymal stem cells in patients with psoriasis. Exp Dermatol 23:521–523
Hou R, Yan H, Niu X, Chang W, An P, Wang C, Yang Y, Yan X, Li J, Liu R et al (2014b) Gene expression profile of dermal mesenchymal stem cells from patients with psoriasis. J Eur Acad Dermatol Venereol: JEADV 28:1782–1791
Hu Y, Chen Z, Gong Y, Shi Y (2018) A review of switching biologic agents in the treatment of moderate-to-severe plaque psoriasis. Clin Drug Investig 38:191–199
Jee MH, Mraz V, Geisler C, Bonefeld CM (2020) γδ T cells and inflammatory skin diseases. Immunol Rev
Kim N, Lee S, Kang J, Choi YA, Lee B, Kwon TK, Jang YH, Kim SH (2020) Hispidulin alleviates imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation. Int Immunopharmacol 87:106767
Küçük C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, Lack N, Alkan C, Williams JC, Avery KN et al (2015) Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun 6:6025
Mak RK, Hundhausen C, Nestle FO (2009) Progress in understanding the immunopathogenesis of psoriasis. Actas Dermosifiliogr 100(Suppl 2):2–13
Nestle FO, Kaplan DH, Barker J (2009) Psoriasis. N Engl J Med 361:496–509
Niu X, Chang W, Liu R, Hou R, Li J, Wang C, Li X, Zhang K (2016a) Expression of pro-angiogenic genes in mesenchymal stem cells derived from dermis of patients with psoriasis. Int J Dermatol 55:e280-288
Niu X, Chang W, Liu R, Hou R, Li J, Wang C, Li X, Zhang K (2016b) mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clin Exp Dermatol 41:533–540
Paganelli A, Tarentini E, Benassi L, Kaleci S, Magnoni C (2020) Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol
Prinz I, Sandrock I (2015) γδ T cells come to stay: innate skin memory in the aldara model. Eur J Immunol 45:2994–2997
Ryan JM, Barry FP, Murphy JM, Mahon BP (2005) Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (London, England) 2:8
Sah SK, Park KH, Yun CO, Kang KS, Kim TY (2016) Effects of human mesenchymal stem cells transduced with superoxide dismutase on imiquimod-induced psoriasis-like skin inflammation in mice. Antioxid Redox Signal 24:233–248
Sato Y, Ogawa E, Okuyama R (2020) Role of innate immune cells in psoriasis. Int J Mol Sci 21
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14:493–507
Song N, Scholtemeijer M, Shah K (2020) Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci
Subedi S, Gong Y, Chen Y, Shi Y (2019) Infliximab and biosimilar infliximab in psoriasis: efficacy, loss of efficacy, and adverse events. Drug Des Devel Ther 13:2491–2502
Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X et al (2010) Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 62:2467–2475
Toussirot E (2012) The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 11:159–168
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP et al (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845
Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P, Schneider J, Perez-Fernandez R (2019) Mesenchymal stem cells in homeostasis and systemic diseases: hypothesis, evidences, and therapeutic opportunities. Int J Mol Sci 20
Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W, Xie L, Lin L, Min J, Lai X et al (2014) Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int J Mol Med 33:263–270
Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, Li Y, Bai W, Li M, Ji H et al (2013) Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells and Development 22:3192–3202
Wang SG, Hsu NC, Wang SM, Wang FN (2020) Successful treatment of plaque psoriasis with allogeneic gingival mesenchymal stem cells: a case study. Case Rep Dermatol Med 2020:4617520
Wu JJ, Valdecantos WC (2017) Adalimumab in chronic plaque psoriasis: a clinical guide. J Drugs Dermatol 16:779–790
Xie XJ, Di TT, Wang Y, Wang MX, Meng YJ, Lin Y, Xu XL, Li P, Zhao JX (2018) Indirubin ameliorates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting inflammatory responses mediated by IL-17A-producing γδ T cells. Mol Immunol 101:386–395
Acknowledgements
Not applicable.
Funding
This work was sponsored by grants from National Natural Science Foundation of China (No. 81872522, 82073429, 81903205, 81803120, and 81900612), the National Key Research and Development Program of China (No. 2018YFC1705301, 2018YFC1705305), Innovation Program of Shanghai Municipal Education Commission (No. 2019–01-07–00-07-E00046), the Program of Science and Technology Commission of Shanghai Municipality (No. 18140901800), Excellent Subject Leader Program of Shanghai Municipal Commission of Health and Family Planning (No. 2018BR30), Clinical Research Program of Shanghai Hospital Development Center (No. SHDC2020CR1014B, SHDC12018X06), Shanghai Sailing Program (No. 19YF1438100), Program of Shanghai Academic Research Leader (No. 20XD1403300), the Fundamental Research Funds for the Central Universities (#22120210566), and Research Program of Shanghai Skin Disease Hospital (No. 2019KYQD08).
Author information
Authors and Affiliations
Contributions
Youdong Chen contributed to conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Yifan Hu was involved in conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, and manuscript writing. Xue Zhou contributed to conception and design, provision of study material, collection and assembly of data, and data analysis and interpretation. Zihan Zhao, Peng Xu, Qian Yu, Zengyang Yu, Zeyu Chen, and Yuanyuan Wang contributed to provision of study material and data analysis and interpretation. Chunyuan Guo was involved in conception and design, financial support, provision of study material, data analysis and interpretation, and final approval of manuscript. Xilin Zhang contributed to provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Yuling Shi contributed to conception and design, financial support, administrative support, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Human and animal rights and informed consent
All mouse studies were approved by the Institutional Animal Experiment Committee of the Tongji University School of Medicine and were performed in accordance with institutional and governmental guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
441_2022_3616_MOESM1_ESM.tif
Supplementary file1 Fig. S1 Schematic of the experimental procedures. a Experimental design of Fig. 2. b Experimental design of Fig. 3. c Experimental design of Fig. 4. d Experimental design of Fig. 5 (TIF 284 KB)
441_2022_3616_MOESM2_ESM.tif
Supplementary file2 Fig. S2 The number of injection points, time points of administration, or injection volumes do not influence the therapeutic effects of hUC-MSCs. a Disease model mice were subcutaneously injected with two million hUC-MSCs on day 0 at one injection point in 500 μl PBS (group A), at five injection points in 500 μl PBS (group B), at one injection point in 200 μl PBS (group C); group D was only given 200 μl PBS at one injection point. The experimental mice were sacrificed after six consecutive days of IMQ application (n = 54, three independent experiments, three mice for each group, and 18 mice for each experiment). b Representative pictures of mouse skin lesions. c Evaluation of PASI score. d H&E staining and calculated epidermal thicknesses e. * indicates comparison with untreated psoriatic group and unpaired t test was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (TIF 986 KB)
441_2022_3616_MOESM3_ESM.tif
Supplementary file3 Fig. S3 hUC-MSC treatment modulates the expression of inflammatory cytokines and chemokines. Mice were treated as in Fig. 5. Dorsal skin samples from control mice, disease model mice, and hUC-MSC-treated IMQ mice were collected, and IL-4, IL-10, IL-6, IL-17, IL-22, IL-23A, IL-1β, TNF-α, IFN-γ, TGF-β, and CXCL-15 mRNA expression was analyzed by quantitative RT-qPCR (n = 48, four independent experiments, three mice for each group and 12 mice for each experiment). The data are the mean ± SEM, and unpaired t test was used for statistical analysis. * indicates comparison with untreated psoriatic group and unpaired t test was used for statistical analysis. *P < 0.05. (TIF 441 KB)
441_2022_3616_MOESM4_ESM.tif
Supplementary file4 Fig. S4 hUC-MSCs do not alter IL-6 and TNF-α protein production. Mice were treated as in Fig. 5. Dorsal skin samples from control mice, disease model mice, and hUC-MSC-treated IMQ mice were collected, and protein levels of IL-6 a and TNF-α b were evaluated quantitatively by ELISA (n = 48, four independent experiments, three mice for each group, and 12 mice for each experiment). The data are the mean ± SEM, and unpaired t test was used for statistical analysis. * indicates comparison with untreated psoriatic group and unpaired t test was used for statistical analysis. *P < 0.05. (TIF 84 KB)
Rights and permissions
About this article
Cite this article
Chen, Y., Hu, Y., Zhou, X. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing γδ T cells. Cell Tissue Res 388, 549–563 (2022). https://doi.org/10.1007/s00441-022-03616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03616-x