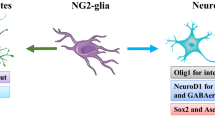
Abstract
NG2 immunopositive progenitor cells, also simply termed as NG2 glia and thought mainly to be oligodendrocyte precursor cells (OPCs), form synaptic connections with neurons in gray and white matters of brain. One of the most classical features of oligodendrocyte lineage cells is myelination, which will favor neuronal signaling transmission. Thus, is there a causal link between the specific synapses of neuron-NG2 glia and myelination? Building on this, here, we will discuss several relevant issues. First, in order to understand the synapses, it is necessary to integrate the definite inputs onto NG2 glia. We show that the synaptic activities and myelination are not synchronized, so the synapses are more likely to regulate early development of NG2 glia and prepare for myelination. Furthermore, several studies have suggested that the synapses also play a role in recovery of pathological conditions, such as multiple sclerosis (MS). Therefore, elucidating the activities of neuron-NG2 glia synapses will be beneficial for both physiological and pathological conditions.

The existence of neuron-NG2 glia synapses reveals that the neuronal activities projecting to NG2 glia is an elaborate regulation, and the signaling from neurons to NG2 glia is frequent in early stage. The neuron-NG2 glia synapses indirectly provide a basic condition to support myelination by extrasynaptic communication. The neuron-NG2 glia synapses also promote remyelination, and it occurs similar to physiological conditions.


Similar content being viewed by others
Abbreviations
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- CNS:
-
Central nervous system
- DPL:
-
Day post lesion
- EPSCs:
-
Excitatory postsynaptic currents
- FSIs:
-
Fast-spiking interneurons
- GABA:
-
γ-Aminobutyric acid
- IPSCs:
-
Inhibitory postsynaptic currents
- MNTB:
-
Medial nucleus of the trapezoid body
- MS:
-
Multiple sclerosis
- NFSIs:
-
Non-fast-spiking interneurons
- NMDA:
-
N-methyl-D-aspartate
- OL:
-
Oligodendrocyte
- OPCs:
-
Oligodendrocyte precursor cells
- PV:
-
Parvalbumin
- SVZ:
-
Subventricular zone
References
Ahrendsen JT, Grewal HS, Hickey SP, Culp CM, Gould EA, Shimizu T, Strnad FA, Traystman RJ, Herson PS, Macklin WB (2016) Juvenile striatal white matter is resistant to ischemia-induced damage. Glia 64(11):1972–1986
Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, Kinney HC (2010) The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol 20(4):803–814
Arellano RO, Sánchez-Gómez MV, Alberdi E, Canedo-Antelo M, Chara JC, Palomino A, Pérez-Samartín A, Matute C (2016) Axon-to-glia interaction regulates GABAA receptor expression in oligodendrocytes. Mol Pharmacol 89(1):63–74
Balia M, Vélez-Fort M, Passlick S, Schäfer C, Audinat E, Steinhäuser C, Seifert G, Angulo MC (2015) Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb Cortex 25(4):1114–1123
Balia M, Benamer N, Angulo MC (2017) A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 65(11):1821–1832
Baraban M, Koudelka S, Lyons DA (2018) Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat Neurosci 21(1):19–23
Bartos M, Elgueta C (2012) Functional characteristics of parvalbumin- and cholecystokinin- expressing basket cells. J Physiol 590(4):669–681
Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161(1):169–186
Bergles DE, Roberts JD, Somogyi P, Jahr CE (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405(6783):187–191
Bergles DE, Jabs R, Steinhäuser C (2010) Neuron-glia synapses in the brain. Brain Res Rev 63(1–2):130–137
Cawley N, Solanky BS, Muhlert N, Tur C, Edden RA, Wheeler-Kingshott CA, Miller DH, Thompson AJ, Ciccarelli O (2015) Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 138(Pt 9):2584–2595
Chen TJ, Kula B, Nagy B, Barzan R, Gall A, Ehrlich I, Kukley M (2018) In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep 25(4):852–861
Chittajallu R, Aguirre A, Gallo V (2004) NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol 561(Pt 1):109–122
Dammann O, Hagberg H, Leviton A (2001) Is periventricular leukomalacia an axonopathy as well as an oligopathy? Pediatr Res 49(4):453–457
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65(1):101–105
De Biase LM, Bergles DE (2011) Same players, different game: AMPA receptor regulation in oligodendrocyte progenitors. Nat Neurosci 14(11):1358–1360
De Biase LM, Nishiyama A, Bergles DE (2010) Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci 30(10):3600–3611
De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE (2011) NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci 31(35):12650–12662
Domercq M, Etxebarria E, Pérez-Samartín A, Matute C (2005) Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia 52(1):36–46
Doyle S, Hansen DB, Vella J, Bond P, Harper G, Zammit C, Valentino M, Fern R (2018) Vesicular glutamate release from central axons contributes to myelin damage. Nat Commun 9(1):1032
Etxeberria A, Mangin JM, Aguirre A, Gallo V (2010) Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci 13(3):287–289
Fannon J, Tarmier W, Fulton D (2015) Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 3(6):1021–1035
Fino E, Yuste R (2011) Dense inhibitory connectivity in neocortex. Neuron 69(6):1188–1203
Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC (1996) Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci 16(8):2659–2670
Gautier HO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJ, Káradóttir RT (2015) Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun 6:8518
Ge WP, Zhou W, Luo Q, Jan LY, Jan YN (2009) Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci U S A 106(1):328–333
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014) Science 344(6183):1252304
Grubman A, Chew G, Ouyang JF, Sun G, Choo XY, McLean C, Simmons RK, Buckberry S, Vargas-Landin DB, Poppe D, Pflueger J, Lister R, Rackham OJL, Petretto E, Polo JM (2019) A single-cell atlas of entorhinal cortex from individuals with Alzheimer's disease reveals cell-type-specific gene expression regulation. Nat Neurosci 22(12):2087–2097
Hamilton NB, Clarke LE, Arancibia-Carcamo IL, Kougioumtzidou E, Matthey M, Káradóttir R, Whiteley L, Bergersen LH, Richardson WD, Attwell D (2017) Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65(2):309–321
Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC (2008) Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res 63(6):656–661
Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B (2015) Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci 18(5):683–689
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345(6196):1255263
Jabs R, Pivneva T, Hüttmann K, Wyczynski A, Nolte C, Kettenmann H, Steinhäuser C (2005) Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci 118(Pt 16):3791–3803
Káradóttir R, Attwell D (2007) Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145(4):1426–1438
Káradóttir R, Cavelier P, Bergersen LH, Attwell D (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 438(7071):1162–1166
Káradóttir R, Hamilton NB, Bakiri Y, Attwell D (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11(4):450–456
Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA (2016) Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr Biol 26(11):1447–1455
Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, Richardson WD (2017) Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife6 e28080
Krasnow AM, Attwell D (2016) NMDA receptors: power switches for oligodendrocytes. Neuron 91(1):3–5
Krasnow AM, Ford MC, Valdivia LE, Wilson SW, Attwell D (2018) Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat Neurosci 21(1):24–28
Kukley M, Capetillo-Zarate E, Dietrich D (2007) Vesicular glutamate release from axons in white matter. Nat Neurosci 10(3):311–320
Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D (2008) Glial cells are born with synapses. FASEB J 22(8):2957–2969
Kukley M, Nishiyama A, Dietrich D (2010) The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci 30(24):8320–8331
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487(7408):443–448
Levine JM, Stincone F, Lee YS (1993) Development and differentiation of glial precursor cells in the rat cerebellum. Glia 7(4):307–321
Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C (2013) A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61(5):732–749
Lin SC, Bergles DE (2004) Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci 7(1):24–32
Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE (2005) Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46(5):773–785
Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Ffrench-Constant C, Attwell D, Káradóttir RT (2013) Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol 11(12):e1001743
Maldonado PP, Angulo MC (2015) Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 21(3):266–276
Maldonado PP, Vélez-Fort M, Angulo MC (2011) Is neuronal communication with NG2 cells synaptic or extrasynaptic? J Anat 219(1):8–17
Mangin JM, Kunze A, Chittajallu R, Gallo V (2008) Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci 28(30):7610–7623
Mangin JM, Li P, Scafidi J, Gallo V (2012) Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci 15(9):1192–1194
Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ, Jensen FE (2008) NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci 28(26):6670–6678
Martinez-Lozada Z, Waggener CT, Kim K, Zou S, Knapp PE, Hayashi Y, Ortega A, Fuss B (2014) Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIβ's actin-binding/−stabilizing domain. Glia 62(9):1543–1558
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM (2003) Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 23(8):3308–3315
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A (2006) Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26(30):7907–7918
Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA (2015) Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 18(5):628–630
Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD (2016) A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 5:e15784
Moshrefi-Ravasdjani B, Dublin P, Seifert G, Jennissen K, Steinhäuser C, Kafitz KW, Rose CR (2017) Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct Funct 222(2):831–847
Mousavi Majd A, Ebrahim Tabar F, Afghani A, Ashrafpour S, Dehghan S, Gol M, Ashrafpour M, Pourabdolhossein F (2018) Inhibition of GABA a receptor improved spatial memory impairment in the local model of demyelination in rat hippocampus. Behav Brain Res 336:111–121
Müller J, Reyes-Haro D, Pivneva T, Nolte C, Schaette R, Lübke J, Kettenmann H (2009) The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol 134(2):115–127
Nagy B, Hovhannisyan A, Barzan R, Chen TJ, Kukley M (2017) Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol 15(8):e2001993
Orduz D, Maldonado PP, Balia M, Vélez-Fort M, de Sars V, Yanagawa Y, Emiliani V, Angulo MC (2015) Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. Elife 4
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458
Passlick S, Grauer M, Schäfer C, Jabs R, Seifert G, Steinhäuser C (2013) Expression of the γ2-subunit distinguishes synaptic and extrasynaptic GABA(A) receptors in NG2 cells of the hippocampus. J Neurosci 33(29):12030–12040
Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Pérez-Samartín A, Pérez-Cerdá F, Bakhtiari D, Matute C, Löwel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave KA (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91(1):119–132
Sahel A, Ortiz FC, Kerninon C, Maldonado PP, Angulo MC, Nait-Oumesmar B (2015) Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Front Cell Neurosci 9:77
Sakry D, Karram K, Trotter J (2011) Synapses between NG2 glia and neurons. J Anat 219(1):2–7
Salter MG, Fern R (2005) NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438(7071):1167–1171
Shen Y, Liu XB, Pleasure DE, Deng W (2012) Axon-glia synapses are highly vulnerable to white matter injury in the developing brain. J Neurosci Res 90(1):105–121
Song FE, Huang JL, Lin SH, Wang S, Ma GF, Tong XP (2017) Roles of NG2-glia in ischemic stroke. CNS Neurosci Ther 23(7):547–553
Stedehouder J, Couey JJ, Brizee D, Hosseini B, Slotman JA, Dirven CMF, Shpak G, Houtsmuller AB, Kushner SA (2017) Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb Cortex 27(10):5001–5013
Stedehouder J, Brizee D, Shpak G, Kushner SA (2018) Activity-dependent myelination of parvalbumin interneurons mediated by axonal morphological plasticity. J Neurosci 38(15):3631–3642
Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, Hisatsune T (2009) Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex 19(9):2181–2195
Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, Angulo MC (2010) Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J Neurosci 30(20):6921–6929
Volpe JJ (2001) Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50(5):553–562
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333(6049):1647–1651
Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD (2015) Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 6:7844
Wang F, Yang YJ, Yang N, Chen XJ, Huang NX, Zhang J, Wu Y, Liu Z, Gao X, Li T, Pan GQ, Liu SB, Li HL, Fancy SPJ, Xiao L, Chan JR, Mei F (2018) Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99(4):689–701
Wise SP, Jones EG (1978) Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J Comp Neurol 178(2):187–208
Xiao L, Hu C, Yang W, Guo D, Li C, Shen W, Liu X, Aijun H, Dan W, He C (2013) NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration. Glia 61(12):2078–2099
Yang QK, Xiong JX, Yao ZX (2013) Neuron-NG2 cell synapses: novel functions for regulating NG2 cell proliferation and differentiation. Biomed Res Int 2013:402843
Yuan X, Eisen AM, McBain CJ, Gallo V (1998) A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125(15):2901–2914
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci 22(5):719–728
Zhu X, Bergles DE, Nishiyama A (2008) NG2 cell generate both oligodendrocytes and gray matter astrocytes. Development 135(1):145–157
Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE (2007) Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10(3):321–330
Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, Dupree JL, Harvey L, Sun D, Hübner CA, Cull-Candy SG, Farrant M, Gallo V (2015) GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat Neurosci 18(5):674–682
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 31371147) and the Innovative Science Project of Chongqing for Graduate Students (No. CYB17136).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Innervation of NG2 glia is similar to a neuron-neuron pattern.
2. The neuron-NG2 glia synapses support myelination indirectly by promoting development of NG2 glia.
3. The regulation pattern altered from synaptic to extrasynaptic communication in late stage.
4. Remyelination may be enhanced by activities of neuron-NG2 glia synapses.
Rights and permissions
About this article
Cite this article
Li, R., Zhang, P., Zhang, M. et al. The roles of neuron-NG2 glia synapses in promoting oligodendrocyte development and remyelination. Cell Tissue Res 381, 43–53 (2020). https://doi.org/10.1007/s00441-020-03195-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03195-9