Abstract
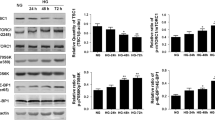
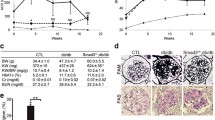
Extracellular matrix deposition during tubulointerstitial fibrosis (TIF), a central pathological process in patients with diabetic nephropathy (DN), is driven by locally activated, disease-relevant myofibroblasts. Myofibroblasts can arise from various cellular sources, e.g., tubular epithelial cells via a process named epithelial-to-mesenchymal transition (EMT). Transforming growth factor beta 1 (TGF-β1) and its downstream Smad signaling play a critical role in both TIF and EMT. Whereas Smad3 is one central mediator, the role of the other prominently expressed variant, Smad2, is not completely understood. In this study, we sought to analyze the role of renal Smad2 in the development of TIF and EMT during streptozotocin-induced DN by using a fibroblast-specific protein 1 (FSP1)-promotor-driven SMAD2 knockout mouse model with decreased tubular, endothelial, and interstitial Smad2 expression. In contrast to wild-type diabetic mice, diabetic SMAD2 knockout mice showed the following features: (1) significantly reduced DN and TIF (shown by KIM1 expression; periodic acid Schiff staining; collagen I and III, fibronectin, and connective tissue growth factor deposition); (2) significantly reduced tubular EMT-like changes (e.g., altered Snail1, E-cadherin, matrix metalloproteinase 2, and vimentin deposition); and (3) significantly decreased expression of myofibroblast markers (α-smooth muscle actin, FSP1). As one mechanism for the protection against diabetes-induced TIF and EMT, decreased Smad3 protein levels and, as a possible consequence, reduced TGF-β1 levels were observed in diabetic SMAD2 knockout mice. Our findings thus support the important role of Smad2 for pro-fibrotic TGF-β/Smad3 signaling in experimental DN.









Similar content being viewed by others
References
Alpers CE, Hudkins KL, Floege J, Johnson RJ (1994) Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol 5:201–209
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303:848–851
Boor P, Floege J (2012) The renal (myo-)fibroblast: a heterogeneous group of cells. Nephrol Dial Transplant 27:3027–3036
Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R (2013) TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24:385–392
Bottinger EP, Bitzer M (2002) TGF-beta signaling in renal disease. J Am Soc Nephrol 13:2600–2610
Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, RA MI, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T, Animal Models of Diabetic Complications C (2009) Mouse models of diabetic nephropathy. J Am Soc Nephrol 20:2503–2512
Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH (2006) Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20:1898–1900
Fujimoto M, Maezawa Y, Yokote K, Joh K, Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y, Mori S (2003) Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun 305:1002–1007
Galichon P, Hertig A (2011) Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair 4:11
Grande MT, Perez-Barriocanal F, Lopez-Novoa JM (2010) Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm 7:19
Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, Rowe RG, Weiss SJ, Lopez-Novoa JM, Nieto MA (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21:989–997
Hammerschmidt E, Loeffler I, Wolf G (2009) Morg1 heterozygous mice are protected from acute renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 297:F1273–F1287
Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC (2008) Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol 19:1584–1591
Herzlinger D (2002) Renal interstitial fibrosis: remembrance of things past? J Clin Invest 110:305–306
Hinz B (2010) The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–155
Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG (2005) Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int 67:2488–2493
Isono M, Chen S, Hong SW, Iglesias-de la Cruz MC, Ziyadeh FN (2002) Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-beta-induced fibronectin in mesangial cells. Biochem Biophys Res Commun 296:1356–1365
Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG (2001) Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther 3:149–159
Ju W, Ogawa A, Heyer J, Nierhof D, Yu L, Kucherlapati R, Shafritz DA, Bottinger EP (2006) Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol Cell Biol 26:654–667
Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, Yoshimura A (2015) Smad2 and Smad3 inversely regulate TGF-beta autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 43:65–79
Kunisch E, Jansen A, Kojima F, Loffler I, Kapoor M, Kawai S, Rubio I, Crofford LJ, Kinne RW (2009) Prostaglandin E2 differentially modulates proinflammatory/prodestructive effects of TNF-alpha on synovial fibroblasts via specific E prostanoid receptors/cAMP. J Immunol 183:1328–1336
Lan A, Qi Y, Du J (2014) Akt2 mediates TGF-beta1-induced epithelial to mesenchymal transition by deactivating GSK3beta/snail signaling pathway in renal tubular epithelial cells. Cell Physiol Biochem 34:368–382
Lan HY (2011) Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci 7:1056–1067
Lan HY (2012) Smads as therapeutic targets for chronic kidney disease. Kidney Res Clin Pract 31:4–11
LeRoy EC, Trojanowska MI, Smith EA (1990) Cytokines and human fibrosis. Eur Cytokine Netw 1:215–219
Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1–12
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7:684–696
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Loeffler I, Wolf G (2015a) Epithelial-to-mesenchymal transition in diabetic nephropathy: fact or fiction? Cell 4:631–652
Loeffler I, Wolf G (2015b) Morg1 heterozygous deficiency ameliorates hypoxia-induced acute renal injury. Am J Physiol Renal Physiol 308:F511–F521
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21:998–1009
Luo J, Liang M, Mitch WE, Danesh FR, Yu M, Cheng J (2015) FSP-1 impairs the function of endothelium leading to failure of arteriovenous grafts in diabetic mice. Endocrinology 156:2200–2210
Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY (2010) Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21:1477–1487
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Menon MC, Ross MJ (2016) Epithelial-to-mesenchymal transition of tubular epithelial cells in renal fibrosis: a new twist on an old tale. Kidney Int 89:263–266
Meran S, Steadman R (2011) Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92:158–167
Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW, American Diabetes A (2004) Nephropathy in diabetes. Diabetes Care 27(Suppl 1):S79–S83
Okada H, Danoff TM, Fischer A, Lopez-Guisa JM, Strutz F, Neilson EG (1998) Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am J Physiol 275:F306–F314
Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG (2000) Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int 58:587–597
Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H (2005) Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16:133–143
Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME (2006) The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J 393:601–607
Phanish MK, Winn SK, Dockrell ME (2010) Connective tissue growth factor (CTGF, CCN2)—a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol 114:e83–e92
Qi W, Chen X, Poronnik P, Pollock CA (2006) The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 38:1–5
Qiao B, Johnson NW, Gao J (2010) Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J Oncol 37:663–668
Schiller M, Javelaud D, Mauviel A (2004) TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 35:83–92
Strutz F, Zeisberg M (2006) Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17:2992–2998
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG (1995) Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130:393–405
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, Heer E de, Joh K, Noel LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology S (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21:556–563
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Tveitaras MK, Skogstrand T, Leh S, Helle F, Iversen BM, Chatziantoniou C, Reed RK, Hultstrom M (2015) Matrix metalloproteinase-2 knockout and heterozygote mice are protected from hydronephrosis and kidney fibrosis after unilateral ureteral obstruction. PLoS One 10:e0143390
Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Nino MD, Sanz AB, Ramos AM, Berzal S, Ruiz-Ortega M, Egido J, Ortiz A (2014) Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol 46:765–776
Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A (2005) TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16:1987–2002
Verrecchia F, Chu ML, Mauviel A (2001) Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 276:17058–17062
Yang J, Liu Y (2001) Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159:1465–1475
Yokoyama K, Kamata N, Fujimoto R, Tsutsumi S, Tomonari M, Taki M, Hosokawa H, Nagayama M (2003) Increased invasion and matrix metalloproteinase-2 expression by snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol 22:891–898
Zeisberg M, Duffield JS (2010) Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol 21:1247–1253
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429–1437
Zhang M, Fraser D, Phillips A (2006) ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. Am J Pathol 169:1282–1293
Acknowledgements
We thank Erwin P. Böttinger for providing the SMAD2 flox/flox mouse line and Eric G. Neilson for providing the FSP1-cre mouse line. We also express our gratitude to Drs. Joachim Clement and Ignacio Rubio for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Loeffler, I., Liebisch, M., Allert, S. et al. FSP1-specific SMAD2 knockout in renal tubular, endothelial, and interstitial cells reduces fibrosis and epithelial-to-mesenchymal transition in murine STZ-induced diabetic nephropathy. Cell Tissue Res 372, 115–133 (2018). https://doi.org/10.1007/s00441-017-2754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2754-1