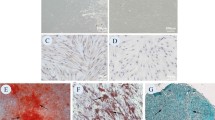
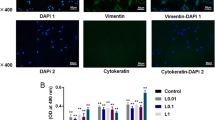
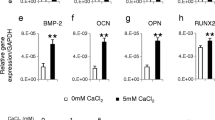
Abstract
Regeneration of periodontal tissues, particularly cementum, is key to regaining periodontal attachment and health. Human periodontal ligament stem cells (hPDLSCs) have been shown to be a good cell source to regenerate periodontal tissues. However, their subpopulations and the differentiation induction in relation to cementogenic lineages is unclear. Thus, we aim to examine the expression of cementum-associated genes in PDLSC subpopulations and determine the effect of broadly used osteogenic stimulus or vitamin C (VC) on the expression of cementogenic and osteogenic genes in PDLSCs. Our real-time quantitative polymerase chain reaction (qPCR) analysis showed that cementogenic marker cementum attachment protein (CAP) expressed only slightly higher in STRO-1+/CD146+, STRO-1−/CD146+ and STRO-1−/CD146− subpopulations than in the original cell pool, while cementum protein 1 (CEMP1) expression in these subpopulations was not different from the original pool. Notably, under the stimulation with osteogenic differentiation medium, CAP and CEMP1 were downregulated while osteogenic markers bone sialoprotein (BSP) and osteocalcin (OCN) were upregulated. Both CAP and CEMP1 were upregulated by VC treatment. Transplantation of VC-treated PDLSCs into immunocompromised mice resulted in forming significantly more ectopic cementum- and bone-like mineral tissues in vivo. Immunohistochemical analysis of the ectopic growth showed that CAP and CEMP1 were mainly expressed in the mineral tissue and in some cells of the fibrous tissues. We conclude that osteogenic stimulation is not inductive but appears to be inhibitory of cementogenic pathways, whereas VC induces cementogenic lineage commitment by PDLSCs and may be a useful stimulus for cementogenesis in periodontal regeneration.






Similar content being viewed by others
Abbreviations
- ALP:
-
Tissue-nonspecific alkaline phosphatase
- BSA:
-
Bovine serum albumin
- BSP:
-
Bone sialophosphoprotein
- CAP:
-
Cementum attachment protein
- CEMP1 or CP23:
-
Cementum protein 1
- DAPI:
-
4’,6-diamidino-2-phenylindole dihydrochloride
- FACS:
-
Fluorescence-activated cell sorting.
- hPDLSCs:
-
Human periodontal ligament stem cells
- MSCs:
-
Mesenchymal stem cells
- OCN:
-
Osteocalcin
- PDL:
-
Periodontal ligament
- RUNX2:
-
Runt-related transcription factor 2
References
Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I (2005) Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res 40:245–251
Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT-J (2010) Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 5:617–631
Alvarez-Pérez MA, Narayanan S, Zeichner-David M, Rodríguez Carmona B, Arzate H (2006) Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 38:409–419
Arzate H, Olson SW, Page RC, Gown AM, Narayanan AS (1992) Production of a monoclonal antibody to an attachment protein derived from human cementum. FASEB J 6:2990–2995
BarKana I, Narayanan AS, Grosskop A, Savion N, Pitaru S (2000) Cementum attachment protein enriches putative cementoblastic populations on root surfaces in vitro. J Dent Res 79:1482–1488
Carmona-Rodríguez B, Álvarez-Pérez MA, Narayanan AS, Zeichner-David M, Reyes-Gasga J, Molina-Guarneros J, García-Hernández AL, Suárez-Franco JL, Chavarría IG, Villarreal-Ramírez E, Arzate H (2007) Human cementum protein 1 induces expression of bone and cementum proteins by human gingival fibroblasts. Biochem Biophys Res Commun 358:763–769
Chen SC, Marino V, Gronthos S, Bartold PM (2006) Location of putative stem cells in human periodontal ligament. J Periodontal Res 41:547–553
D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ (1997) Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone 20:117–126
Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S (2010) Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 28:1829–1838
Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S (2010) Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis 16:20–28
Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, Ishikawa I (2008) Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res 43:364–371
Gay I, Chen S, MacDougall M (2007) Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofacial Res 10:149–160
Hasegawa T, Chosa N, Asakawa T, Yoshimura Y, Ishisaki A, Tanaka M (2010) Establishment of immortalized human periodontal ligament cells derived from deciduous teeth. Int J Mol Med 26:701–705
Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A 99:9445–9449
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249
Hoz L, Romo E, Zeichner-David M, Sanz M, Nunez J, Gaitan L, Mercado G, Arzate H (2012) Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol Int 36:129–136
Huang GTJ, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806
Huang GT-J, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010) Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16:605–615
Isaka J, Ohazama A, Kobayashi M, Nagashima C, Takiguchi T, Kawasaki H, Tachikawa T, Hasegawa K (2001) Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72:314–323
Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, Okano T, Ishikawa I (2009) Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 30:2716–2723
Jaha H, Husein D, Ohyama Y, Xu D, Suzuki S, Huang GT, Mochida Y (2016) N-terminal dentin sialoprotein fragment induces type I collagen production and upregulates dentinogenesis marker expression in osteoblasts. Biochem Biophys Rep 6:190–196
Komaki M, Iwasaki K, Arzate H, Narayanan AS, Izumi Y, Morita I (2012) Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J Cell Physiol 227:649–657
Liao J, Al Shahrani M, Al-Habib M, Tanaka T, Huang GT (2011) Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod 37:1217–1224
Liu HW, Yacobi R, Savion N, Narayanan AS, Pitaru S (1997) A collagenous cementum-derived attachment protein is a marker for progenitors of the mineralized tissue-forming cell lineage of the periodontal ligament. J Bone Miner Res 12:1691–1699
Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH (2005) In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim 41:232–238
Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S (2008) Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 2007–0734
Melcher AH (1976) On the repair potential of periodontal tissues. J Periodontol 47:256–260
Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S (2014) Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev 23:1001–1011
Metzger Z, Weinstock B, Dotan M, Narayanan AS, Pitaru S (1998) Differential chemotactic effect of cementum attachment protein on periodontal cells. J Periodontal Res 33:126–129
Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S (2013) Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A
Romanos GE, Asnani KP, Hingorani D, Deshmukh VL (2014) PERIOSTIN: role in formation and maintenance of dental tissues. J Cell Physiol 229:1–5
Saygin NE, Giannobile WV, Somerman MJ (2000) Molecular and cell biology of cementum. Periodontol 24:73–98
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S (2005) Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res 84:907–912
Torii D, Konishi K, Watanabe N, Goto S, Tsutsui T (2015) Cementogenic potential of multipotential mesenchymal stem cells purified from the human periodontal ligament. Odontology 103:27–35
Tour G, Wendel M, Moll G, Tcacencu I (2012) Bone repair using periodontal ligament progenitor cell-seeded constructs. J Dent Res 91:789–794
Tsai MT, Li WJ, Tuan RS, Chang WH (2009) Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res 27:1169–1174
Viereck V, Siggelkow H, Tauber S, Raddatz D, Schutze N, Hufner M (2002) Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem 86:348–356
Villarreal-Ramírez E, Moreno A, Mas-Oliva J, Chávez-Pacheco JL, Narayanan AS, Gil-Chavarría I, Zeichner-David M, Arzate H (2009) Characterization of recombinant human cementum protein 1 (hrCEMP1): primary role in biomineralization. Biochem Biophys Res Commun 384:49–54
Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, Liu Y, Zhang C, Shi S, Wang S (2012) Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol 227:3216–3224
Wei F, Song T, Ding G, Xu J, Liu Y, Liu D, Fan Z, Zhang C, Shi S, Wang S (2013) Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev 22:1752–1762
Yu Z, Gauthier P, Tran QT, El Ayachi I, Bhatti F-U-R, Bahabri R, Al-Habib M, Huang GTJ (2015) Differential properties of human ALP+ periodontal ligament stem cells vs their ALP- counterparts. J Stem Cell Res Ther 5:292
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health R01 DE019156 (G.T.-J.H.), by an Endodontic Research Grant from the American Association of Endodontists Foundation (G.T.-J.H.) and a Research Fund from the University of Tennessee Health Science Center.
Authors’ contributions
PG and ZY designed and performed the experimental work, acquired, assembled, analyzed the data and drafted/revised the manuscript. QTT analyzed, interpreted the data, performed statistical analysis and wrote/revised part of the manuscript. FURB and XZ performed some of the experimental work, assembled, analyzed the data and drafted/revised part of the manuscript. GTJH conceived, designed, performed experimental works and supervised the overall project, analyzed and interpreted the data and finalized the manuscript. All authors have read and approved the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Philippe Gauthier and Zongdong Yu contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1007/s00441-016-2550-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 25 kb)
ESM 2
(DOCX 14 kb)
ESM 3
(DOCX 18 kb)
ESM 4
(DOCX 12 kb)
ESM 5
(DOCX 11 kb)
ESM 6
(DOCX 11 kb)
ESM 7
(DOCX 11 kb)
Supplemental Fig. 1
Measurement of in vivo mineral tissue formation by PDLSCs. (a) A low -magnification view of a section showing the entire mass of the resected tissue containing aggregated HA/TCP granules encapsulated by the subcutaneous fibrous connective tissue. The image was analyzed by ImageJ to calculate the total area of the mineral tissues that include the HA/TCP granules, newly formed mineral tissues and small amounts of fibrous tissues among the mineral tissues. Manual outlining the total area with ImageJ to allow quantitation of the outlined area was carefully performed as indicated by the encircled black dashed line. Some samples that shown large areas (similar to the size of a granule) of soft tissues (encircled by the red dashed lines) within the tissue mass were excluded. Sample from a control group. Scale bar: 1 mm. (b) A closer view of the mineral tissues showing scattered newly formed mineral tissues on the surface of HA/TCP granules. These mineral tissues were manually encircled (white dashed line) and numbered with ImageJ and the areas calculated. All the small mineral areas calculated were summed together as the area of newly formed mineral tissues. Sample from a VC treated group. Scale bar: 100 μm. HA: HA/TCP (GIF 723 kb)
Supplemental Fig. 2
RT-PCR showing gene expression of cementum markers (CAP, CEMP1) and osteogenic markers (ALP, RUNX2, BSP, OCN) in PDLSCs cultured in GM as controls (C) and in OM (O) after 0, 3, 7, 10, 15 and 21 days. Data from cells derived from one donor. (GIF 111 kb)
Supplemental Fig. 3
PDLSC cultures with or without osteogenic stimulation. (a, a’, a”) PDLSCs isolated from PDL at p0 with regular GM. Scale bars: a, 400 μm; a’, 200 μm; a”, 100 μm. (b, b’) PDLSCs from two different donors b, b’, under OM stimulation for 3 weeks with mineral deposits (more amount and diffused in b’). Scale bar: 50 μm for both b & b’. (c) PDLSCs under OM stimulation for 2 or 4 weeks (wks) and stained with Alizarin Red S. Scale bar: 50 μm. (GIF 193 kb)
Supplemental Fig. 4
PDLSC cultures with or without VC stimulation. (a-d) PDLSCs stimulation for 3 weeks in regular GM with (b, d) or without (a, c) 20 μg/mL VC. (a, b are from a donor; c, d are from a different donor and the cells were used for mouse transplantation experiment – data in Fig. 5). Scale bar: 100 μm for all 4 images. (GIF 364 kb)
Supplemental Fig. 5
Mineral tissue formation in vivo by PDLSCs. Sample sources and experimental conditions are the same as those in Fig. 5. (a-c) Less calcified mineral structures with less organized fiber orientations. Blue arrowheads indicate Sharpey’s fiber-like structures. (d-e) More calcified mineral tissue with lamellar lines (red arrowheads). Ctrl: cells grown in regular medium; VC: cells treated with VC. (a, b, e) From donor 1 VC group; (c, d) from donor 3 Ctrl group; (f) from donor 3 VC group. Scale bar: (a, b) 50 μm for both images. (c-f) 50 μm for all 4 images. (GIF 686 kb)
Supplemental Fig. 6
Immunohistochemical analysis of tissue regenerated in vivo by PDLSCs. Sample sources and experimental conditions are the same as those in Fig. 6. HA: hydroxyapatite/tri-calcium phosphate; M: mineral tissue; Ct: fibrous connective tissue. Arrows: in CAP group, blue arrows indicate mineral tissues with brown staining, red arrows indicate Sharpey’s fiber-like structures; in CEMP1 group, blue arrows indicate mineral tissues with brown staining, red arrows indicate immunoreactivities in cells of the fibrous connective tissues; in Mito (mitochondria) group, red arrows indicate stronger staining in cells lining against the mineral tissue and weaker staining in cells in the adjacent soft connective tissue; in Ctrl-IgG group, blue arrows indicate mineral tissue. Ctrl-IgG group: Top image is pre-immune mouse IgG isotype control, bottom image is pre-immune rabbit IgG isotype control. Scale bars: in CAP and CEMP1, 300 μm for left images, 50 μm for right images; in Mito, 50 μm for all 4 images; in Ctrl-IgG, 100 μm for both images. (GIF 1389 kb)
Rights and permissions
About this article
Cite this article
Gauthier, P., Yu, Z., Tran, Q.T. et al. Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res 368, 79–92 (2017). https://doi.org/10.1007/s00441-016-2513-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2513-8