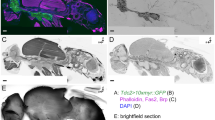
Abstract
The monoamines octopamine and tyramine, which are the invertebrate counterparts of epinephrine and norepinephrine, transmit their action through sets of G protein-coupled receptors. Four different octopamine receptors (Oamb, Octß1R, Octß2R, Octß3R) and 3 different tyramine receptors (TyrR, TyrRII, TyrRIII) are present in the fruit fly Drosophila melanogaster. Utilizing the presumptive promoter regions of all 7 octopamine and tyramine receptors, the Gal4/UAS system is utilized to elucidate their complete expression pattern in larvae as well as in adult flies. All these receptors show strong expression in the nervous system but their exact expression patterns vary substantially. Common to all octopamine and tyramine receptors is their expression in mushroom bodies, centers for learning and memory in insects. Outside the central nervous system, the differences in the expression patterns are more conspicuous. However, four of them are present in the tracheal system, where they show different regional preferences within this organ. On the other hand, TyrR appears to be the only receptor present in the heart muscles and TyrRII the only one expressed in oenocytes. Skeletal muscles express octß2R, Oamb and TyrRIII, with octß2R being present in almost all larval muscles. Taken together, this study provides comprehensive information about the sites of expression of all octopamine and tyramine receptors in the fruit fly, thus facilitating future research in the field.









Similar content being viewed by others
References
Adamo SA, Linn CE, Hoy RR (1995) The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J Exp Biol 198:1691–1700
Balfanz S, Strunker T, Frings S, Baumann A (2005) A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem 93:440–451
Bayliss A, Roselli G, Evans PD (2013) A comparison of the signalling properties of two tyramine receptors from Drosophila. J Neurochem 125:37–48
Beggs KT, Tyndall JD, Mercer AR (2011) Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 6:e26809
Blenau W, Baumann A (2001) Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48:13–38
Blenau W, Rademacher E, Baumann A (2012) Plant essential oils and formamidines as insecticides/acaricides. What are the molecular targets? Apidologie 43:344–347
Blumenthal EM (2003) Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 284:C718–C728
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415
Braunig P, Burrows M (2004) Projection patterns of posterior dorsal unpaired median neurons of the locust subesophageal ganglion. J Comp Neurol 478:164–175
Brembs B, Christiansen F, Pfluger HJ, Duch C (2007) Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J NeuroscI 27:11122–11131
Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S (2012) Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492:433–437
Busch S, Tanimoto H (2010) Cellular configuration of single octopamine neurons in Drosophila. J Comp Neurol 518:2355–2364
Busch S, Selcho M, Ito K, Tanimoto H (2009) A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol 513:643–667
Cabrero P, Richmond L, Nitabach M, Davies SA, Dow JA (2013) A biogenic amine and a neuropeptide act identically: tyramine signals through calcium in Drosophila tubule stellate cells. Proc R Soc Lond B 280:20122943
Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ (2005) A new family of insect tyramine receptors. Biochem Biophys Res Commun 338:1189–1196
Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39:715–720
Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65:670–681
Darriba D, Taboada GL, Doallo R, Posada D (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27:1164–1165
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Erion R, DiAngelo JR, Crocker A, Sehgal A (2012) Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem 287:32406–32414
Evans PD, Maqueira B (2005) Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci 5:111–118
Evans PD, Robb S (1993) Octopamine receptor subtypes and their modes of action. Neurochem Res 18:869–874
Faisal MN, Hoffmann J, El-Kholy S, Kallsen K, Wagner C, Bruchhaus I, Fink C, Roeder T (2014) Transcriptional regionalization of the fruit fly’s airway epithelium. PLoS ONE 9:e102534
Fink C, Staubach F, Kuenzel S, Baines JF, Roeder T (2013) Noninvasive analysis of microbiome dynamics in the fruit fly Drosophila melanogaster. Appl Environ Microbiol 79:6984–6988
Fox LE, Soll DR, Wu CF (2006) Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J Neurosci 26:1486–1498
Gutierrez E, Wiggins D, Fielding B, Gould AP (2007) Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445:275–280
Han KA, Millar NS, Davis RL (1998) A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci 18:3650–3658
Hoffmann J, Romey R, Fink C, Yong L, Roeder T (2013) Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging 5:315–327
Hoyle G (1975) Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J Exp Zool 193:425–431
Hoyle G, Barker DL (1975) Synthesis of octopamine by insect dorsal median unpaired neurons. J Exp Zool 193:433–439
Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, Iyer N, Fetter D, Hausenfluck JH, Peng H, Trautman ET, Svirskas RR, Myers EW, Iwinski ZR, Aso Y, DePasquale GM, Enos A, Hulamm P, Lam SC, Li HH, Laverty TR, Long F, Qu L, Murphy SD, Rokicki K, Safford T, Shaw K, Simpson JH, Sowell A, Tae S, Yu Y, Zugates CT (2012) A GAL4-driver line resource for Drosophila neurobiology. Cell Rep 2:991–1001
Kim YC, Lee HG, Lim J, Han KA (2013) Appetitive learning requires the alpha1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. J Neurosci 33:1672–1677
Lee HG, Seong CS, Kim YC, Davis RL, Han KA (2003) Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol 264:179–190
Li Y, Fink C, El-Kholy S, Roeder T (2015) The octopamine receptor octß2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch Insect Biochem & Physiol 88:168–178
Lim J, Sabandal PR, Fernandez A, Sabandal JM, Lee HG, Evans P, Han KA (2014) The octopamine receptor Octbeta2R regulates ovulation in Drosophila melanogaster. PLoS ONE 9:e104441
Maqueira B, Chatwin H, Evans PD (2005) Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem 94:547–560
Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V (1995) Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol 356:275–287
Monastirioti M, Linn CE Jr, White K (1996) Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci 16:3900–3911
Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV (2013) Factors that regulate insulin producing cells and their output in. Front Physiol 4:252
Rahn T, Leippe M, Roeder T, Fedders H (2013) EGFR signaling in the brain is necessary for olfactory learning in Drosophila larvae. Learn Mem 20:194–200
Roeder T (1992) A new octopamine receptor class in locust nervous tissue, the octopamine 3 (OA3) receptor. Life Sci 50:21–28
Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59:533–561
Roeder T (2003) Metabotropic histamine receptors–nothing for invertebrates? Eur J Pharmacol 466:85–90
Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50:447–477
Roeder T, Degen J, Dyczkowski C, Gewecke M (1995) Pharmacology and molecular biology of octopamine receptors from different insect species. Prog Brain Res 106:249–258
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rotte C, Krach C, Balfanz S, Baumann A, Walz B, Blenau W (2009) Molecular characterization and localization of the first tyramine receptor of the American cockroach (Periplaneta americana). Neuroscience 162:1120–1133
Saraswati S, Fox LE, Soll DR, Wu CF (2004) Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol 58:425–441
Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R (1990) Cloning and characterization of a Drosophila tyramine receptor. EMBO J 9:3611–3617
Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M (2003) Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23:10495–10502
Selcho M, Pauls D, El Jundi B, Stocker RF, Thum AS (2012) The role of octopamine and tyramine in Drosophila larval locomotion. J Comp Neurol 520:3764–3785
Selcho M, Pauls D, Huser A, Stocker RF, Thum AS (2014) Characterization of the octopaminergic and tyraminergic neurons in the central brain of Drosophila larvae. J Comp Neurol (in press)
Sharma Y, Cheung U, Larsen EW, Eberl DF (2002) PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in drosophila. Genesis 34:115–118
Sinakevitch I, Mustard JA, Smith BH (2011) Distribution of the octopamine receptor AmOA1 in the honey bee brain. PLoS ONE 6:e14536
Sinakevitch IT, Smith AN, Locatelli F, Huerta R, Bazhenov M, Smith BH (2013) Apis mellifera octopamine receptor 1 (AmOA1) expression in antennal lobe networks of the honey bee (Apis mellifera) and fruit fly (Drosophila melanogaster). Front Syst Neurosci 7:70
Sombati S, Hoyle G (1984) Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol 15:481–506
Suver MP, Mamiya A, Dickinson MH (2012) Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr Biol 22:2294–2302
Zeng H, Loughton BG, Jennings KR (1996) Tissue specific transduction systems for octopamine in the locust (Locusta migratoria). J Insect Physiol 42:765–769
Zhou C, Rao Y, Rao Y (2008) A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 11:1059–1067
Zornik E, Paisley K, Nichols R (1999) Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 20:45–51
Acknowledgments
We would like to thank Britta Laubenstein for excellent technical assistance. This work was supported by the DFG (Sonderforschungsbereich Transregio TR22, project A07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Kholy, S., Stephano, F., Li, Y. et al. Expression analysis of octopamine and tyramine receptors in Drosophila . Cell Tissue Res 361, 669–684 (2015). https://doi.org/10.1007/s00441-015-2137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2137-4