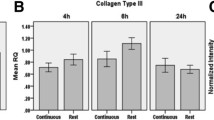
Abstract
Tendon calcification is common in the Achilles tendon, and injuries affect not only athletes, but also the general population. However, the underlying cellular mechanisms are not yet fully understood. In this study, we isolated healthy human tenocytes and subjected them to uniaxial mechanical stretching (at 1.0 Hz) for various stretch times (4 h, 8 h, 12 h) or magnitudes (0 %, 4 %, 8 %, 12 %). The extracellular calcium chelator EGTA, calcium channel inhibitor MnCl2, nifedipine, or various doses of exogenous calcium were administered to these cells with or without mechanical overloading. The intracellular calcium concentration was determined by using a Fluo-3/AM fluorescence probe, and the cytoskeleton was revealed by F-actin Phalloidin staining. The intracellular calcium concentration increased in a magnitude- and time-dependent manner following stretching. These increases were suppressed by EGTA, MnCl2, or nifedipine. Additionally, cytoskeleton F-actin was disrupted significantly by stretching in a time-dependent manner. When extracellular calcium was applied, the intracellular calcium concentration increased, and F-actin was disrupted dramatically under mechanical stretching compared with non-stretched cells. Thus, repetitive mechanical overloading induces the accumulation of abnormally high concentrations of intracellular calcium resulting from extracellular calcium influx mediated, at least in part, by membrane calcium channels and finally causes cytoskeleton disorganization and tenocyte dysfunction. These findings provide novel experimental evidence for the pathology of tendon calcification and indicate that the blockade of calcium influx is a potential target for the prevention and treatment of calcific tendinopathy.





Similar content being viewed by others
References
Bjur D, Danielson P, Alfredson H, Forsgren S (2008) Presence of a non-neuronal cholinergic system and occurrence of up- and down-regulation in expression of M2 muscarinic acetylcholine receptors: new aspects of importance regarding Achilles tendon tendinosis (tendinopathy). Cell Tissue Res 331:385–400
Cao D, Liu W, Wei X, Xu F, Cui L, Cao Y (2006) In vitro tendon engineering with avian tenocytes and polyglycolic acids: a preliminary report. Tissue Eng 12:1369–1377
Chao YH, Tsuang YH, Sun JS, Chen LT, Chiang YF, Wang CC, Chen MH (2008) Effects of shock waves on tenocyte proliferation and extracellular matrix metabolism. Ultrasound Med Biol 34:841–852
Crockett RJ, Centrella M, McCarthy TL, Grant Thomson J (2010) Effects of cyclic strain on rat tail tenocytes. Mol Biol Rep 37:2629–2634
Dyachenko V, Husse B, Rueckschloss U, Isenberg G (2009) Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45:38–54
Endlich N, Sunohara M, Nietfeld W, Wolski EW, Schiwek D, Kranzlin B, Gretz N, Kriz W, Eickhoff H, Endlich K (2002) Analysis of differential gene expression in stretched podocytes: osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J 16:1850–1852
Geiger RC, Taylor W, Glucksberg MR, Dean DA (2006) Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther 13:725–731
Goldman J, Zhong L, Liu SQ (2003) Degradation of alpha-actin filaments in venous smooth muscle cells in response to mechanical stretch. Am J Physiol Heart Circ Physiol 284:H1839–H1847
Hayabuchi Y, Nakaya Y, Mawatari K, Inoue M, Sakata M, Kagami S (2011) Cell membrane stretch activates intermediate-conductance Ca2+−activated K+ channels in arterial smooth muscle cells. Heart Vessel 26:91–100
Kilinc D, Gallo G, Barbee KA (2009) Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol 219:553–561
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698
Koo SS, Parsley BK, Burkhart SS, Schoolfield JD (2011) Reduction of postoperative stiffness after arthroscopic rotator cuff repair: results of a customized physical therapy regimen based on risk factors for stiffness. Arthroscopy 27:155–160
Kraichely RE, Strege PR, Sarr MG, Kendrick ML, Farrugia G (2009) Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am J Physiol Gastrointest Liver Physiol 296:G833–G839
Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH (2004) Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med 32:435–440
Lozupone E, Favia A, Grimaldi A (1992) Effect of intermittent mechanical force on bone tissue in vitro: preliminary results. J Bone Miner Res 7:S407–S409
Lu L, Oswald SJ, Ngu H, Yin FC (2008) Mechanical properties of actin stress fibers in living cells. Biophys J 95:6060–6071
Magra M, Hughes S, El Haj AJ, Maffulli N (2007) VOCCs and TREK-1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol 292:C1053–C1060
Miura M, Wakayama Y, Sugai Y, Kagaya Y, Watanabe J, Keurs HE ter, Shirato K (2001) Effect of transient stretch on intracellular Ca2+ during triggered propagated contractions in intact trabeculae. Can J Physiol Pharmacol 79:68–72
Mohanty MJ, Li X (2002) Stretch-induced Ca(2+) release via an IP(3)-insensitive Ca(2+) channel. Am J Physiol Cell Physiol 283:C456–C462
Nakamura TY, Iwata Y, Sampaolesi M, Hanada H, Saito N, Artman M, Coetzee WA, Shigekawa M (2001) Stretch-activated cation channels in skeletal muscle myotubes from sarcoglycan-deficient hamsters. Am J Physiol Cell Physiol 281:C690–C699
Oh KJ, Yoon JR, Shin DS, Yang JH (2010) Extracorporeal shock wave therapy for calcific tendinitis at unusual sites around the hip. Orthopedics 33:769
Oliva F, Via AG, Maffulli N (2011) Calcific tendinopathy of the rotator cuff tendons. Sports Med Arthrosc 19:237–243
Oliva F, Via AG, Maffulli N (2012) Physiopathology of intratendinous calcific deposition. BMC Med 10:95
Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, Wildemann B (2010) Characterization of tendon cell cultures of the human rotator cuff. Eur Cell Mater 20:84–97
Qi MC, Hu J, Zou SJ, Han LC, Luo E (2005) The changes of cytoskeleton F-actin in rat bone marrow mesenchymal stem cells and calvarial osteoblasts under mechanical strain. Hua Xi Kou Qiang Yi Xue Za Zhi 23:110–112, 121 [in Chinese]
Ren Y, Zhan Q, Hu Q, Sun B, Yang C, Wang C (2009) Static stretch induces active morphological remodeling and functional impairment of alveolar epithelial cells. Respiration 78:301–311
Rui YF, Lui PP, Chan LS, Chan KM, Fu SC, Li G (2011) Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Am J Chin Med 124:606–610
Ruwhof C, Laarse A van der (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47:23–37
Sabeti-Aschraf M, Gonano C, Nemecek E, Cichocki L, Schueller-Weidekamm C (2010) Intra-operative ultrasound facilitates the localization of the calcific deposit during arthroscopic treatment of calcifying tendinitis. Knee Surg Sports Traumatol Arthrosc 18:1792–1794
Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M (2004) Cultivation of human tenocytes in high-density culture. Histochem Cell Biol 122:219–228
Siegal DS, Wu JS, Newman JS, Del Cura JL, Hochman MG (2009) Calcific tendinitis: a pictorial review. Can Assoc Radiol J 60:263–272
Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM (1996) Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Physiol 270:C1075–C1087
Stricker J, Falzone T, Gardel ML (2010) Mechanics of the F-actin cytoskeleton. J Biomech 43:9–14
Thampatty BP, Im HJ, Wang JH (2006) Leukotriene B4 at low dosage negates the catabolic effect of prostaglandin E2 in human patellar tendon fibroblasts. Gene 372:103–109
Thampatty BP, Li H, Im HJ, Wang JH (2007) EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene 386:154–161
Vico L, Lafage-Proust MH, Alexandre C (1998) Effects of gravitational changes on the bone system in vitro and in vivo. Bone 22:95S–100S
Wall ME, Banes AJ (2005) Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Nueronal Interact 5:70–84
Wang JH (2006) Mechanobiology of tendon. J Biomech 39:1563–1582
Wang JH, Jia F, Gilbert TW, Woo SL (2003a) Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech 36:97–102
Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL (2003b) Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res 44:128–133
Wang JH, Yang G, Li Z, Shen W (2004) Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J Biomech 37:573–576
Wang JH, Yang G, Li Z (2005) Controlling cell responses to cyclic mechanical stretching. Ann Biomed Eng 33:337–342
Wang JH, Iosifidis MI, Fu FH (2006) Biomechanical basis for tendinopathy. Clin Orthop Relat Res 443:320–332
Wei B, Chen Z, Zhang X, Feldman M, Dong XZ, Doran R, Zhao BL, Yin WX, Kotlikoff MI, Ji G (2008) Nitric oxide mediates stretch-induced Ca2+ release via activation of phosphatidylinositol 3-kinase-Akt pathway in smooth muscle. PLoS One 3:e2526
Wen L, Wang Y, Wang H, Kong L, Zhang L, Chen X, Ding Y (2012) L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 424:439–445
Xu F, Liu W, Cao DJ, ZhaiI HL, Wei X, Cui L, Cao YL (2004) Comparison of types I and III collagen expression in human tendon and skin as well as tynocytes and dermal fibroblasts. Acta Univ Med Secondae Shanghai 24:275–278
Yang G, Im HJ, Wang JH (2005) Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363:166–172
Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW (2008) Role of the calcium-calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol 105:352–357
Acknowledgments
The microgroove silicon membrane was kindly provided by Dr. James H.-C. Wang (University of Pittsburgh, USA) who also strictly supervised the custom-made controlling apparatus. We are grateful to Dr. Kaifa Wang (Third Military Medical University, Chongqing, China) for kind assistance with the statistical analysis and to Yang Xiang (Central Laboratory of Southwest Hospital, Chongqing, China) for the collection and measurement of the morphological data from confocal microscopy.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wan Chen and Yinshuan Deng contributed equally to this study.
This work was supported by the Natural Science Foundation of China (81230040, 30872620, and 81071464) and Chongqing Science and Technology Committee (CSTC2011BA5010).
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Material Figure S1
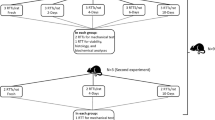
The uniaxial cyclic mechanical stretching device with the microgroove silicon membrane system. a The cyclic mechanical stretch device. b This device is set at 4%, 8%, and 12% magnitude (two dishes each) at 1.0 Hz or 0.5 Hz. c The tension unit. d The device in an incubator. (GIF 178 kb)
Rights and permissions
About this article
Cite this article
Chen, W., Deng, Y., Zhang, J. et al. Uniaxial repetitive mechanical overloading induces influx of extracellular calcium and cytoskeleton disruption in human tenocytes. Cell Tissue Res 359, 577–587 (2015). https://doi.org/10.1007/s00441-014-2018-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-2018-2