Abstract
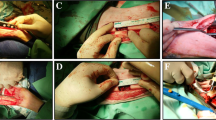
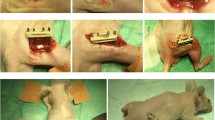
Tissue engineering provides new potential treatments for the repair of bone defects. Bone-marrow-derived mesenchymal stem cells (BMSCs) represent an attractive cell source for therapeutic applications involving tissue engineering, although disadvantages, such as pain of harvest and low proliferation efficiency, are major limitations to the application of BMSCs in the clinic. Adipose-derived stem cells (ASCs) with their multilineage potential and satisfactory proliferation potential can be induced into the osteogenic lineage in vitro and can be anchored onto suitable scaffolds as seed cells to repair bone defects successfully in an autologous setting. Previous studies have indicated that both undifferentiated BMSCs and ASCs exhibit immunosuppression and immunoprivilege properties. We compare the immuno-function of undifferentiated and osteo-differentiated ASCs in vitro and explore the feasibility of applying allogeneic ASCs to the repair of ulnar bone defects in the rabbit model. Our study demonstrates that allogeneic osteogenic differentiated ASCs maintain low immunogenicity and negative immunomodulation. The allogeneic osteogenic differentiated ASCs combined with demineralized bone matrix successfully regenerate ulnar bone defects in rabbits without immunosuppressive therapies.










Similar content being viewed by others
References
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363–408
Buschmann J, Gao S, Härter L, Hemmi S, Welti M, Werner CM, Calcagni M, Cinelli P, Wanner GA (2013) Yield and proliferation rate of adipose-derived stromal cells as a function of age, body mass index and harvest site—increasing the yield by use of adherent and supernatant fractions? Cytotherapy 15:1098–1105
Castillo M, Liu K, Bonilla L, Rameshwar P (2007) The immune properties of mesenchymal stem cells. Int J Biomed Sci 3:76–80
Colson YL, Tripp RA, Doherty PC, Wren SM, Neipp M, Abou El-Ezz AY, Ildstad ST (1998) Antiviral cytotoxic activity across a species barrier in mixed xenogeneic chimeras: functional restriction to host MHC. J Immunol 160:3790–3796
Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22:560–567
Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y (2007a) Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 28:5477–5486
Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y (2007b) Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng 13:1185–1195
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109
Ding DC, Chou HL, Hung WT, Liu HW, Chu TY (2013) Human adipose-derived stem cells cultured in keratinocyte serum free medium: donor’s age does not affect the proliferation and differentiation capacities. J Biomed Sci 20:59
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24:150–154
Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO (2012) Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 64:1063–1077
Gu H, Guo F, Zhou X, Gong L, Zhang Y, Zhai W, Chen L, Cen L, Yin S, Chang J, Cui L (2011) The stimulation of osteogenic differentiation of human adipose-derived stem cells by ionic products from akermanite dissolution via activation of the ERK pathway. Biomaterials 32:7023–7033
Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, Sakai D, Kudoh T, Kawamoto K, Eguchi H, Satoh T, Tanemura M, Nagano H, Doki Y, Mori M, Ishii H (2013) Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ 55:309–318
Le Blanc K (2003) Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 5:485–489
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31:890–896
Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O (2004) Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 60:307–315
Liu G, Zhang Y, Liu B, Sun J, Li W, Cui L (2013) Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials 34:2655–2664
Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T (2006) The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol 176:2864–2871
Liu Q, Cen L, Zhou H, Yin S, Liu G, Liu W, Cao Y, Cui L (2009) The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng Part A 15:3487–3497
Mizuno H (2009) Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch 76:56–66
Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U (2007) Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng 13:111–121
O’Keefe RJ, Mao J (2011) Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Eng Part B Rev 17:389–392
Ren ML, Peng W, Yang ZL, Sun XJ, Zhang SC, Wang ZG, Zhang B (2012) Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant 21:2711–2721
Shegarfi H, Reikeras O (2009) Review article: bone transplantation and immune response. J Orthop Surg (Hong Kong) 17:206–211
Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100:157–168
Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A (2013) Comparison of mesenchymal stem cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 15:434–448
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Author information
Authors and Affiliations
Corresponding authors
Additional information
Huijie Gu and Zhuyou Xiong contributed equally to this work.
This work was financially supported by the Natural Science Foundation of China (grant nos. 81201204, 81301335) and the Education Department of Anhui Province Natural Science Research Project (grant no. KJ2010A239).
Rights and permissions
About this article
Cite this article
Gu, H., Xiong, Z., Yin, X. et al. Bone regeneration in a rabbit ulna defect model: use of allogeneic adipose-derivedstem cells with low immunogenicity. Cell Tissue Res 358, 453–464 (2014). https://doi.org/10.1007/s00441-014-1952-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1952-3