Abstract
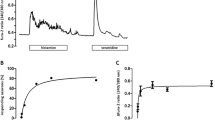
We recently observed a bradykinin-induced increase in the cytosolic Ca2+ concentration in submucosal neurons of rat colon, an increase inhibited by blockers of voltage-dependent Ca2+ (Cav) channels. As the types of Cav channels used by this part of the enteric nervous system are unknown, the expression of various Cav subunits has been investigated in whole-mount submucosal preparations by immunohistochemistry. Submucosal neurons, identified by a neuronal marker (microtubule-associated protein 2), are immunoreactive for Cav1.2, Cav1.3 and Cav2.2, expression being confirmed by reverse transcription plus the polymerase chain reaction. These data agree with previous observations that the inhibition of L- and N-type Ca2+ currents strongly inhibits the response to bradykinin. However, whole-cell patch-clamp experiments have revealed that bradykinin does not enhance Ca2+ inward currents under voltage-clamp conditions. Consequently, bradykinin does not directly interact with Cav channels. Instead, the kinin-induced Ca2+ influx is caused indirectly by the membrane depolarization evoked by this peptide. As intracellular Ca2+ channels on Ca2+-storing organelles can also contribute to Ca2+ signaling, their expression has been investigated by imaging experiments and immunohistochemistry. Inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) have been functionally demonstrated in submucosal neurons loaded with the Ca2+-sensitive fluorescent dye, fura-2. Histamine, a typical agonist coupled to the phospholipase C pathway, induces an increase in the fura-2 signal ratio, which is suppressed by 2-aminophenylborate, a blocker of IP3 receptors. The expression of IP3R1 has been confirmed by immunohistochemistry. In contrast, ryanodine, tested over a wide concentration range, evokes no increase in the cytosolic Ca2+ concentration nor is there immunohistochemical evidence for the expression of ryanodine receptors in these neurons. Thus, rat submucosal neurons are equipped with various types of high-voltage activated Cav channels and with IP3 receptors for intracellular Ca2+ signaling.








Similar content being viewed by others
References
Avemary J, Diener M (2010a) Effects of bradykinin B2 receptor stimulation at submucosal ganglia from rat distal colon. Eur J Pharmacol 627:295–303
Avemary J, Diener M (2010b) Bradykinin-induced depolarization and Ca2+ influx through voltage-gated Ca2+ channels in rat submucosal neurons. Eur J Pharmacol 635:87–95
Barbado M, Fablet K, Ronjat M, De Waard M (2009) Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta 1793:1096–1104
Bkaily G, Avedanian L, Jacques D (2009) Nuclear membrane receptors and channels as targets for drug development in cardiovascular diseases. Can J Physiol Pharmacol 87:108–119
Blondel O, Takeda J, Janssen H, Seino S, Bell GI (1993) Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem 268:11356–11363
Bödding M (2001) Histamine-induced Ca2+ release in bovine adrenal chromaffin cells. Naunyn-Schmiedeberg’s Arch Pharmacol 364:508–515
Bringmann A, Schopf S, Reichenbach A (2000) Developmental regulation of calcium channel-mediated currents in retinal glial (Müller) cells. J Neurophysiol 84:2975–2983
Cai W, Hisatsune C, Nakamura K, Nakamura T, Inoue T, Mikoshiba K (2004) Activity-dependent expression of inositol 1,4,5-trisphosphate receptor type 1 in hippocampal neurons. J Biol Chem 279:23691–23698
Catterall WA, Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59:882–901
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425
Choi JY, Beaman-Hall CM, Vallano ML (2004) Granule neurons in cerebellum express distinct splice variants of the inositol trisphosphate receptor that are modulated by calcium. Am J Physiol 287:C971–C980
Franklin JL, Willard AL (1993) Voltage-dependent sodium and calcium currents of rat myenteric neurons in cell culture. J Neurophysiol 69:1264–1275
García-Palomero E, Cuchillo-Ibáñez I, García A, Renart J, Albillos A, Montiel C (2000) Greater diversity than previously thought of chromaffin cell Ca2+ channels, derived from mRNA identification studies. FEBS Lett 481:235–239
Harris RA, Hanrahan JW (1993) Histamine stimulates a biphasic calcium response in the human tracheal epithelial cell line CF/T43. Am J Physiol 265:C781–C791
Haschke G, Schäfer KH, Diener M (2002) Effect of butyrate on membrane potential, ionic currents and intracellular Ca2+-concentration in cultured rat myenteric neurons. Neurogastroenterol Motil 14:133–142
Kim MB, Lee SH, Shim WS, Oh U (2004) Histamine-induced Ca2+ influx via the PLA2/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett 361:159–162
Kirchgessner AL, Liu MT (1999) Differential localization of Ca2+ channel α1 subunits in the enteric nervous system: presence of α1B channel-like immunoreactivity in intrinsic primary afferent neurons. J Comp Neurol 409:85–104
Kocks S, Schultheiss G, Diener M (2002) Ryanodine receptors and the mediation of Ca2+-dependent anion secretion across rat colon. Pflügers Arch Eur J Physiol 445:390–397
Köhler R, Brakemeier S, Kühn M, Degenhardt C, Buhr H, Pries A, Hoyer J (2001) Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res 51:160–168
Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM (2006) C-termini of P/Q-type Ca2.1 channel α1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet 15:1587–1599
Kruglov EA, Gautam S, Guerra MT, Nathanson MH (2011) Type 2 inositol 1,4,5-trisphosphate receptor modulates bile salt export pump activity in rat hepatocytes. Hepatology 54:1790–1799
Li CH (2008) A reliable whole cell clamp technique. Adv Physiol Educ 32:209–211
Lin Z, Sandgren K, Ekblad E (2004) Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton Neurosci 114:29–38
Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G (2002) α1C (CaV1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283:C1001–C1008
Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba M (1997) 2-APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122:498–505
McPherson PS, Campbell KP (1993) Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. J Biol Chem 268:19785–19790
Mikoshiba K (2006) Inositol 1,4,5-trisphosphate (IP3) receptors and their role in neuronal cell function. J Neurochem 97:1627–1633
Motagally MA, Neshat S, Lomax AE (2009) Inhibition of sympathetic N-type voltage-gated Ca2+ current underlies the reduction in norepinephrine release during colitis. Am J Physiol 296:G1077–G1084
Nahm SS, Farnell YZ, Griffith W, Earnest DJ (2005) Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci 25:9304–9308
Newton CL, Mignery GA, Sudhof TC (1994) Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem 269:28613–28619
Pierobon N, Renard-Rooney DC, Gaspers LD, Thomas AP (2006) Ryanodine receptors in liver. J Biol Chem 281:34086–34095
Prinz G, Diener M (2008) Characterization of ryanodine receptors in rat colonic epithelium. Acta Physiol 193:151–162
Rehn M, Hübschle T, Diener M (2004) TNF-alpha hyperpolarizes membrane potential and potentiates the response to nicotinic receptor stimulation in cultured rat myenteric neurons. Acta Physiol Scand 181:13–22
Sharp AH, Nucifora FC Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA (1999) Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol 406:207–220
Siefjediers A, Hardt M, Prinz G, Diener M (2007) Characterization of inositol 1,4,5-trisphosphate (IP3) receptor subtypes at rat colonic epithelium. Cell Calcium 41:303–315
Sitmo M, Rehn M, Diener M (2007) Stimulation of voltage-dependent Ca2+ channels by NO at rat myenteric neurons. Am J Physiol Gastrointest Liver Physiol 293:G886–G893
Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN (2009) N-type and P/Q-type calcium channels regulate differentially the release of noradrenaline, ATP and β-NAD in blood vessels. Neuropharmacology 56:368–378
Stephens GJ, Morris NP, Fyffe REW, Robertson B (2001) The Cav2.1/α1A (P/Q- type) voltage-dependent calcium channel mediates inhibitory neurotransmission onto mouse cerebellar Purkinje cells. Eur J Neurosci 13:1902–1912
Stutzmann GE, Mattson MP (2011) Endoplasmic reticulum Ca2+ handling in excitable cells in health and disease. Pharmacol Rev 63:700–727
Taylor CW, Genazzani AA, Morris SA (1999) Expression of inositol trisphosphate receptors. Cell Calcium 26:237–251
Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP (1988) Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci 11:431–438
Verkhratsky A, Kettenmann H (1996) Calcium signalling in glia cells. Trends Neurosci 19:346–352
Vinet J, Sík A (2006) Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience 143:189–212
Wood JD (1997) Physiology of the enteric nervous system. In: Johnson LR (ed) Physiology of the gastrointestinal tract, vol 1, 3rd edn. Raven, New York, pp 67–109
Xu JH, Long L, Wang J, Tang YC, Hu HT, Soong TW, Tang FR (2010) Nuclear localization of Cav2.2 and its distribution in the mouse central nervous system, and changes in the hippocampus during and after pilocarpine-induced status epilepticus. Neuropathol Appl Neurobiol 36:71–85
Yang S, Huang XY (2005) Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem 280:27130–27137
Zhang M, Sukiasyan N, Møller M, Bezprozvanny I, Zhang H, Wienecke J, Hultborn H (2006) Localization of L-type calcium channel Cav1.3 in cat lumbar spinal cord—with emphasis on motoneurons. Neurosci Lett 407:42–47
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft (grant Di 388/13-1).
Rights and permissions
About this article
Cite this article
Rehn, M., Bader, S., Bell, A. et al. Distribution of voltage-dependent and intracellular Ca2+ channels in submucosal neurons from rat distal colon. Cell Tissue Res 353, 355–366 (2013). https://doi.org/10.1007/s00441-013-1643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1643-5