Abstract
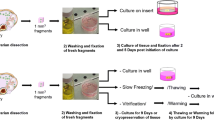
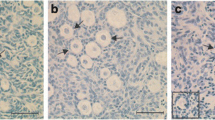
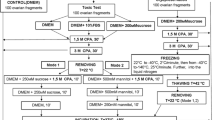
Goat ovarian cortex fragments were subjected to slow freezing in the presence of various solutions containing intracellular cryoprotectants, including 1.0 M ethylene glycol (EG), propanediol (PROH), or dimethyl sulfoxide (DMSO), with or without sucrose and/or fetal calf serum (FCS). Histological examination revealed that only the DMSO-containing solutions were able to maintain a follicular ultrastructure similar to the morphology observed in the fresh control. Therefore, fragments previously cryopreserved in DMSO solutions (with and without sucrose and/or FCS) were cultured in vitro for 48 h and then subjected to viability, histological, and ultrastructural analysis. No significant differences were observed among the percentages of morphologically normal follicles in cryopreserved ovarian tissue before in vitro culture (DMSO: 62.5%; DMSO + sucrose: 68.3%; DMSO + FCS: 60.0%; DMSO + sucrose + FCS: 60.0%) and after culture (DMSO: 60.8%; DMSO + sucrose: 64.2%; DMSO + FCS: 70.8%; DMSO + sucrose + FCS: 55.0%). Following in vitro culture, the viability analysis showed that only the freezing solution containing DMSO and FCS (75.6%) maintained a percentage of viable follicles similar to that observed after culture without cryopreservation (89.3%). As determined by ultrastructural analysis, morphologically normal preantral follicles were detected in the fresh control and in fragments cultured before and after cryopreservation with DMSO and FCS. Thus, a freezing solution containing DMSO and FCS, under the experimental conditions tested here, guaranteed the maintenance of viability and follicular ultrastructure after short-term in vitro culture.





Similar content being viewed by others
References
Abedelahi A, Salehnia M, Allameh AA (2008) The effects of different concentration of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J Assist Reprod Genet 25:483–488
Amorim CA, Langendonckt AV, David A, Dolmans MM, Donnez J (2009) Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro cultures in a calcium alginate matrix. Hum Reprod 24:92–99
Anchordoguy T, Carpenter JF, Loomis SH, Crowe JH (1988) Mechanisms of interaction of amino acids with phospolipid bilayers during freezing. Biochim Biophys Acta 946:299–306
Basso AC, Garcia JM, Esper CR (2007) Efeitos de diferentes sistemas de dultivo in vitro sobre o crescimento de folículos pré-antrais isolados de ovários de fetos bovinos. Braz J Vet Res Anim Sci 44:134–143
Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G (2006) Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod 21:512–517
Broecke RVD, Liu J, Handyside A, Elst JCVD, Krausz T, Dhont M, Winston RM, Hovatta O (2001) Follicular growth in fresh and cryopreserved human ovarian cortical grafts transplanted to immunodeficient mice. Eur J Obstet Gynecol Reprod Biol 97:193–201
Campbell LH, Brockbank KGM (2007) Serum-free solution for cryopreservation of cells. In vitro Cell Dev Biol 43:269–275
Choi JY, Lee BE, Lee EY, Yoon BK, Bae DS, Choi DS (2008) Cryopreservation of ovarian tissue temporarily suppresses the proliferation of granulose cells in mouse preantral follicles. Cryobiology 56:36–42
Comizzole P, Mermillod P, Mauget R (2000) Reproductive biotechnologies for endangered mammalian species. Reprod Nutr Dev 40:493–504
Crowe JH, Tablin F, Wolkers WF, Gousset K, Tsvetkova NM, Ricker J (2003) Stabilization of membranes in human platelets freeze-dried with trehalose. Chem Phys Lipids 122:41–52
Danso KE, Ford-Lloyd BV (2004) Cryopreservation of embryogenic calli of cassava using sucrose cryoprotection and air desiccation. Plant Cell Rep 22:623–631
Fabbri R, Pasquinelli G, Keane D, Magnani V, Paradisi R, Venturoli S (2010) Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod Biomed Online 221:819–828
Faustino LR (2009) Dissertation: Different concentrations and time of exposure to ethylene glycol for cryopreservation of ovarian tissue goat and sheep. Graduate Program in Veterinary Science. State University of Ceara, Fortaleza
Faustino LR, Santos RR, Silva CMG, Pinto LC, Celestino JJH, Campello CC, Figueiredo JR, Rodrigues APR (2010) Goat and sheep ovarian tissue cryopreservation: effects on the morphology and development of primordial follicles and density of stromal cell. Anim Reprod Sci 122:90–97
He S, Woods LC (2004) Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 48:254–262
Huang J, Li Q, Zhao R, Li W, Han Z, Chen X, Xiao B, Shuyun W, Jiang Z, Hu J, Liu L (2008) Effect of sugar on maturation rate of vitrified-thawed immature porcine oocytes. Anim Reprod Sci 106:25–35
Isachenko E, Isachenko V, Rahimi G, Nawroth F (2003) Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Eur J Obstet Gynecol Reprod Biol 1008:186–193
Izadyar F, Matthijs-Rijsenbilt JJ, Ouden KD, Creemers LB, Woelders H, De Rooij DG (2002) Development of a cryopreservation protocol for type A spermatogonia. J Androl 23:537–545
Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S (2007) Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod 22:1060–1067
Lima AK, Silva AR, Santos RR, Sales DM, Evangelista AF, Figueiredo JR, Silva LD (2006) Cryopreservation of preantral ovarian follicles in situ from domestic cats (Felis catus) using different cryoprotective agents. Theriogenology 66:1664–1666
Lucci CM, Amorim CA, Báo SN, Figueiredo JR, Rodrigues APR, Silva JR, Gonçalves PBD (1999) Effect of the interval of serial sections of ovarian in the tissue chopper on the number of isolated caprine preantral follicles. Anim Reprod Sci 56:39–49
Lucci CM, Kacinskis MA, Lopes LHR, Rumpf R, Báo SN (2004) Effect of different cryoprotectants on the structural preservation of follicles in frozen zebu bovine (Bos indicus) ovarian tissue. Theriogenology 61:1101–1114
Luz VB, Santos RR, Pinto LC, Soares AAX, Celestino JJH, Mafezoli J, Campello CC, Figueiredo JR, Rodrigues APR (2009) Dimethyl sulfoxide perfusion in caprine ovarian tissue and its relationship with follicular viability after cryopreservation. Fertil Steril 91:1513–1515
Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN (2002) Effects of culture medium, serum type, and various concentration of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod 67:1197–1203
Marco-Jiménez F, Garzón DL, Peñaranda DS, Pérez L, Viudes-de Castro MP, Vicente JS, Jover M, Asturiano JF (2006) Cryopreservation of European eel (Anguilla anguilla) spermatozoa: effect of dilution ratio, foetal bovine serum supplementation, and cryoprotectants. Cryobiology 53:51–57
Marsella T, Sena P, Xella S, La Marca A, Giulini S, De Pol A, Volpe A, Marzona L (2008) Human ovarian tissue cryopreservation: effect of sucrose concentration on morphological features after thawing. Reprod Biomed Online 16:257–267
Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Masse L, Mousset-Siméon N, Macé B, Rives N (2008) Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod 23:17–28
Onions VJ, Mitchell MRP, Campbell BK, Webb R (2008) Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod 23:606–618
Picton HM, Harris SE, Muruvi W, Chambers EL (2008) The in vitro growth and maturation of follicles. Reproduction 136:703–715
Pinto LC, Santos RR, Faustino LR, Silva CMG, Luz VB, Maia-Júnior JE, Soares AAX, Celestino JJH, Mafezoli J, Campello CC, Figueiredo JR, Rodrigues APR (2008) Quantification of dimethyl sulfoxide perfusion in sheep ovarian tissue: a predictive parameter for follicular survival to cryopreservation. Biopreservation Biobanking 6:269–276
Rodrigues APR, Amorim CA, Costa SHF, Matos MHT, Santos RR, Lucci CM, Báo SN, Ohashi OM, Figueiredo JR (2004) Cryopreservation of caprine ovarian tissue using dimethylsulphoxide and propanediol. Anim Reprod Sci 84:211–227
Santos IRI (2000) Criopreservação: potencial e perspectivas para a conservação de germoplasma vegetal. Rev Bras Fisiol Veg 12:70–84
Santos RR, Rodrigues AP, Costa SH, Silva JR, Matos MH, Lucci CM, Báo SN, Hurk R van den, Figueiredo JR (2006a) Histological and ultrastructural analysis of cryopreserved sheep preantral follicles. Anim Reprod Sci 91:249–263
Santos RR, Tharasanit T, Figueiredo JR, Van Haeften T, Hurk R van den (2006b) Preservation of caprine preantral follicle viability after cryopreservation in sucrose and ethylene glycol. Cell Tissue Res 325:523–531
Santos RR, Knijn HM, Vos PLAM, Oei CHY, Loon T van, Colenbrander B, Gadella BM, Hurk R van den, Roelen BAJ (2009) Complete follicular development and recovery of ovarian function of frozen-thawed, autotransplanted caprine ovarian cortex. Fertil Steril 91:1455–1458
Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O (2011) Clinical grade vitrification of human ovarian tissue: an ultrastuctural analysis of follicles and stroma in vitrifie tissue. Hum Reprod (in press)
Siebzehnrubl E, Kohl J, Dittrich R, Wildt L (2000) Freezing of human ovarian tissue—not the oocytes but the granulose in the problem. Mol Cell Endocrinol 169:109–111
Smith GD, Silva E, Silva CA (2004) Developmental consequences of cryopreservation of mammalian oocytes and embryos. Reprod Biomed Online 9:171–178
Zhang JM, Liu XL, Yang YX, Wan XP (2010) Comparisons of different protocols for vitrifying mouse ovarian tissue. Reprod Domest Anim 45:694–698
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, S.V., de Carvalho, A.A., da Silva, C.M.G. et al. Freezing solution containing dimethylsulfoxide and fetal calf serum maintains survival and ultrastructure of goat preantral follicles after cryopreservation and in vitro culture of ovarian tissue. Cell Tissue Res 346, 283–292 (2011). https://doi.org/10.1007/s00441-011-1257-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1257-8