Abstract
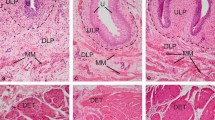
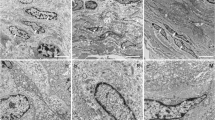
Interstitial cells (ICs) play a role in regulating normal bladder activity. This study explores the possibility that the sub-urothelial and muscle networks of NO/cGMP-responsive ICs are altered in animals with surgically induced outflow obstruction. In sham-operated animals, the urothelium comprised NO-stimulated cGMP-positive (cGMP+) umbrella cells, an intermediate layer and a basal layer that stained for nNOS. cGMP+ sub-urothelial interstitial cells (su-ICs) were found below the urothelium. cGMP+ cells were also associated with the outer muscle layers: on the serosal surface, on the surface of the muscle bundles and within the muscle bundles. Several differences were noted in tissues from obstructed animals: (1) the number of cGMP+ umbrella cells and intensity of staining was reduced; (2) the intermediate layer of the urothelium consisted of multiple cell layers; (3) the su-IC layer was increased, with cells dispersed being throughout the lamina propria; (4) cGMP+ cells were found within the inner muscle layer forming nodes between the muscle bundles; (5) the number of cells forming the muscle coat (serosa) was increased; (6) an extensive network of cGMP+ cells penetrated the muscle bundles; (7) cGMP+ cells surrounded the muscle bundles and nodes of ICs were apparent, these nodes being associated with nerve fibres; (8) nerves were found in the lamina propria but rarely associated with the urothelium. Thus, changes occur in the networks of ICs following bladder outflow obstruction. These changes must have functional consequences, some of which are discussed.











Similar content being viewed by others
References
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Andersson K-E, Yoshida M (2003) Antimuscarinics and the over active detrusor—which is the main mechanism of action? Eur Urol 43:1–5
Birder LA, Kanai AJ, Groat WC de, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ (2001) Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98:13396–13401
Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ (2002) Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22:8063–8070
Brading AF, McCloskey KD (2005) Mechanisms of disease: specialised interstitial cells of the urinary tract—an assessment of knowledge. Nat Clin Pract Urol 2:546–554
Charlton RG, Morley AR, Chambers P, Gillespie JI (1999) Focal changes in nerve, muscle and connective tissue in normal and unstable human bladder. Br J Urol 84:953–960
Chiavegato A, Roelofs M, Franch R, Castellucci E, Sarinella F, Sartore S (1999) Differential expression of SM22 isoforms in myofibroblasts and smooth muscle cells from the rabbit bladder. J Muscle Res Cell Mot 20:133–146
Davidson RA, McCloskey KD (2005) Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol 173:1385–1390
de Groat WC (2004) The urothelium in overactive bladder: passive bystander or active participant. Urology 65:7–11
de Vente J, Hopkins DA, Markerink-van Ittersum M, Emson PC, Schmidt HHHW, Steinbusch HWM (1998) Distribution of nitric oxide synthase and nitric oxide-receptive, cyclic GMP-producing structures in the rat brain. Neuroscience 87:207–241
Drake MJ, Hedlund P, Mills IM, McCoy R, McMurray G, Gardner BP, Andersson K-E, Brading A (2000) Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest 80:199–207
Drake MJ, Harvey IJ, Gillespie JI (2003) Autonomous activity in the isolated guinea pig bladder. Exp Physiol 88:19–30
Drake MJ, Fry CH, Eyden B (2006) Structural characterisation of myofibroblasts in the bladder. Br J Urol Int 97:29–32
Ferguson DR (1999) Urothelial function. Br J Urol Int 84:235–242
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol (Lond) 505:503–511
Finney SM, Andersson K-E, Gillespie JI, Stewart LH (2006) Antimuscarinic drugs in detrusor overactivity and the overactive bladder (OAB) syndrome: motor or sensory actions? Br J Urol Int 98:503–507
Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP (2004) Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology 63 (Suppl 1):24–31
Gillespie JI (2004) The autonomous bladder: a view of the origin of bladder overactivity. Br J Urol Int 93:478–483
Gillespie JI (2005) Animal models of urge incontinence: an evolving view of the origins of bladder overactivity and sensory urge. Br J Urol Int 96:22–28
Gillespie JI, Harvey IJ, Drake MJ (2003) Agonist and nerve induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88:343–357
Gillespie JI, Markerink-van Ittersum M, de Vente J (2004) cGMP generating cells in the bladder wall: identification of distinct networks of interstitial cells. Br J Urol Int 94:1114–1124
Gillespie JI, Markerink-van Ittersum M, de Vente J (2005a) The expression of neuronal nitric oxide synthase (nNOS) and nitric oxide induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321:341–351
Gillespie JI, Markerink-van Ittersum M, de Vente J (2006a) Endogenous NO/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325:325–332
Gillespie JI, Markerink-van Ittersum M, de Vente J (2006b) Interstitial cells and cholinergic signalling in the outer muscle layers of the guinea pig bladder. Br J Urol Int 97:379–385
Gillespie JI, M Markerink-van Ittersum M, de Vente J (2006c) Sensory collaterals, intra-mural ganglia and motor nerves in the guinea pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325:33–45
Hashitani H, Yanai Y, Suzuki H (2004) Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol (Lond) 559:567–581
Iggo A (1955) Tension receptors in the stomach and urinary bladder. J Physiol (Lond) 128:593–607
Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, Groat W de, Fry CH (2007) Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292:F1065–F1072
Lagou M, Drake MJ, Gillespie JI (2005) Volume-induced effects on the isolated bladder: a possible local reflex. Br J Urol Int 94:1356–1365
Lagou M, Drake MJ, Vente J de, Markerink-van Ittersum M, Gillespie JI (2006) The role of interstitial cells in the isolated mouse bladder. Br J Urol Int 98:643–650
Maggi CA (1992) Prostanoids as local modulators of reflex micturition. Pharmacological Res 25:13–20
McCloskey KD, Gurney AM (2002) kit-positive cells in the guinea pig bladder. J Urol 168:832–836
Mikhailidis DP, Jeremy JY, Dandona P (1987) Urinary bladder prostanoids—their synthesis, function and possible role in the pathogenesis and treatment of disease. J Urol 137:577–582
Mostwin JL, Omer M, Karim A, Koeveringe G van, Broks EL (1991) The guinea pig as a model of gradual urethral obstruction. J Urol 145:854–858
Pampinella F, Roelofs M, Castellucci E, Passerini-Glazel G, Pagano F, Sartore S (1997) Time dependent remodelling of the bladder wall in growing rabbits after partial outlet obstruction. J Urol 157:677–682
Roelofs M, Faggian L, Pampinella F, Paulon T, Franch R, Chiavegato A, Sartore S (1998) Transforming growth factor b1 involvement in the conversion of fibroblasts to smooth muscle cells in the rabbit bladder serosa. Histochem J 30:393–404
Smet PJ, Jonavicius J, Marshall VR, de Vente J (1996) Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71:337–348
Starling EH (1905) Elements of human physiology, 7th edn. Churchill, London
Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ (2002) Gap junctions and connexin expression in human suburothelial interstitial cells. Br J Urol Int 90:118–129
Tanaka J, Markerink-van Ittersum M, Steinbusch HWM, de Vente J (1997) Nitric oxide-mediated cGMP synthesis in oligodendrocytes in the developing rat brain. Glia 19:286–297
Vaughan CW, Satchell PM (1995) Urine storage mechanisms. Prog Neurobiol 46:215–237
Wiseman OJ, Brady CM, Hussain IF, Dasgupta P, Watt H, Fowler CJ, Landon DN (2002) The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol 168:2040–2045
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Acknowledgement
We are grateful to Dr. Dennis Oerlemans for his technical support, advice and help with the operations to obstruct the guinea-pig bladders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jongh, R., van Koeveringe, G.A., van Kerrebroeck, P.E.V. et al. Alterations to network of NO/cGMP-responsive interstitial cells induced by outlet obstruction in guinea-pig bladder. Cell Tissue Res 330, 147–160 (2007). https://doi.org/10.1007/s00441-007-0454-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0454-y