Abstract
Demonstration of the importance of the paired box transcription factor Pax7 for the murine myosatellite cell population, with persistent expression in mature skeletal muscle, prompted us to investigate the distribution of Pax7 protein in biopsy samples of normal and pathological human skeletal limb muscle. Immunostaining for M-cadherin, an adhesion molecule present at the interface between myofibre and satellite cell, and the characteristic position adjacent to the muscle fibre and beneath the fibre’s basement membrane were used to identify satellite cells. Anti-Pax7 reactivity was found in the majority of satellite cells but a small population was Pax7 negative. Neither could we identify Pax7-positive nuclei in freshly regenerating myotubes or in presumed myoblasts in these biopsies. Similarly, in myogenic cell cultures derived from the explantation of human foetal muscle Pax7 expression was low or undetectable at the proliferative myoblast stage but it became prominent in an increasing proportion of mononucleate cells after the induction of differentiation. This expression was, however, restricted to mononucleate cells; it did not persist into the differentiation stage of newly formed multinucleate myotubes. Despite this, in the biopsy samples, we occasionally found Pax7-positive nuclei in muscle fibres that seemed to be undergoing degenerative changes. Most of these were found to be the nuclei of cells engaged in focal regenerative processes, but Pax7 re-expression by myonuclei “in distress” cannot be ruled out entirely.
Similar content being viewed by others
Introduction
The Pax family of transcription factors shares the common motif of the paired box DNA binding domain and is known to play important roles in patterning and cell fate determination during embryonal development (for a review, see Mansouri et al. 1996). While some of the Pax proteins are very similar, and the binding of more than one Pax protein to a given paired box binding site has been demonstrated (Holst et al. 1994), they are not fully redundant. Comparison between the splotch (Pax3−) and the Pax7−/− mouse demonstrates that although the proteins are highly similar and each plays a role in limb muscle development, neither compensates fully for the other. While in the splotch mouse the muscle precursors do not migrate to the limb bud for lack of Pax3 driven c-met receptor expression (Epstein et al. 1996), the Pax7−/− mouse does develop limb muscles but these lack satellite cells (Seale et al. 2000). Consequently, Pax7 has been envisaged as essential for the formation of the satellite cell pool in skeletal muscle. As the indispensable role of the satellite cells is to provide new myonuclei for muscle growth and regeneration, these mice are unable to grow or regenerate their limb muscles (Seale et al. 2000). However, this principle might not apply to every single skeletal muscle (Buckingham et al. 2003). Pax7 is not at all muscle specific, it plays a role in the development of the central nervous system and its timely repression is essential for the correct development of motoneuronal precursors (Ericson et al. 1996).
Important as satellite cells are for the maintenance of functional skeletal muscle and thus a focus of attention as a possible key to myoblast transplantation (Heslop et al. 2001; Zammit et al. 2002) and stem cell based therapies (LaBarge and Blau 2002), their identification in tissue is still problematic. Morphologically defined as mononuclear myogenic reserve cells located alongside the muscle fibre underneath the fibre’s basal lamina, their identification with the electron microscope is tedious and restricts both sample size and the use of methods for gaining other types of information. Various immunohistochemical “markers” have been suggested, but few of them are reliable and these still require the additional information of the morphology and histological location of the marked cell. The calcium-dependent transmembrane cell-cell adhesion molecule M-cadherin (Donalies et al. 1991) is one such marker known to be very reliable at least in murine muscle (Irintchev et al. 1994; Rosenblatt et al. 1999). But since it is expressed on all myogenic cells, additional morphological information is needed for specific identification of satellite cells, especially in pathological conditions. However, as the longitudinal extent of a human satellite cell nucleus is greater than 8 µm (Watkins and Cullen 1988), individual satellite cells can be re-identified on serial transverse sections. Therefore, we investigated the pattern of reactivity with anti-Pax7 antibody in relation to anti-M-cadherin (Mcad) and anti-laminin reactivity on serial cryosections both of normal human muscle and of muscle biopsies obtained from patients suffering from a variety of neuromuscular conditions in order to gain insight into the distribution of Pax7 protein.
Materials and methods
Muscle material
Muscle specimens were obtained by standard open biopsy during diagnostic work-up for neuromuscular complaints. The data for the individual patients can be found in Table 1. In addition, we used the specimens of: (1) a tibialis posterior muscle from a 37-year-old male suffering from a dysferlinopathy and of (2) a leg muscle of a 2-year-old girl with as yet unresolved neuromuscular complaints (Fig. 3). These latter muscles were not included in the quantitative analysis but used for additional material to extend and verify the observations, made on the main group of muscles, on muscle spindles and Pax7 positive nuclei inside myofibres. Normal control samples are those of patients whose muscle was ultimately judged not to be abnormal by a combination of clinical, electrophysiological and histological criteria. The muscles were frozen in melting isopentane and stored in liquid nitrogen. Cryosections were cut at 6 µm thickness and transferred to silaned glass slides. The sections were then fixed in a 1:1 methanol acetone mixture for 5 min at –20°C and air dried either to be stained directly or stored at –20°C for subsequent staining. Sections/coverslips that were to be stained with the anti-Myf5 antibody (Santa Cruz Biotechnology, Heidelberg, Germany) received an additional 10 min fixation in 4% paraformaldehyde in PBS at room temperature followed by rinsing with PBS immediately before staining.
Immunostaining
Where goat secondary antibodies were used, the sections were blocked by preincubation for 30 min in 20% normal goat serum (Sigma-Aldrich, Gillingham, UK) in PBS at room temperature. The primary antibodies and their dilutions in PBS were: (1) mouse monoclonal anti-Pax7 (undiluted tissue culture supernatant of hybridoma cells obtained from the Developmental Studies Hybridoma Bank, Iowa, USA), (2) rabbit polyclonal anti-M-cadherin (Irintchev et al. 1994; 1:50), (3) mouse monoclonal anti-laminin (1:1,000; clone LAM-89, Sigma-Aldrich), (4) rabbit polyclonal anti-collagen type IV (1:500; Rockland, Gilbertsville, USA), (5) rabbit polyclonal anti-Myf5 (1:50; Santa Cruz Biotechnology), (6) mouse monoclonal anti-MyoD1 (1:100; clone 5.8A, DAKO, DakoCytomation, Ely, UK), (7) mouse-monoclonal anti-myogenin (1:50; clone F5D, DAKO), (8) FITC-conjugated mouse monoclonal anti-PCNA (1:200; clone PC10, DAKO), (9) polyclonal rabbit anti-desmin (1:300; DAKO), and (10) mouse monoclonal anti-neonatal myosin heavy chain (1:50; clone WB-MHCn, Novocastra, Vector Laboratories, Peterborough, UK). All primary antibodies were incubated overnight at 4°C. PBS was used for washes between the different incubation steps.
Where more than one murine primary antibody was to be applied, the first primary antibody was incubated overnight, followed by a wash and incubation with its secondary antibody for 1 h at room temperature. The remaining binding capacity of this first secondary antibody was then blocked by incubation with 10% normal mouse serum in PBS for 1 h at room temperature. Where the second primary antibody was directly conjugated with a fluorochrome, it was applied in the required dilution in PBS overnight. When the second primary, however, had to be detected by another secondary antibody, a different protocol was used. In that case, the primary and the secondary antibody were incubated together in their respective staining concentrations in PBS in an Eppendorf tube for 5 min at room temperature. Then a tenth of that volume in mouse serum was added to block the remaining free binding sites of the secondary antibody and to avoid the formation of large antibody complexes. This mixture of primary antibody, secondary antibody and serum was left for another 5 min at room temperature before being applied to the sections freshly blocked in 10% normal mouse serum in PBS (see above) and incubated at 4°C overnight. Such double stains with mouse monoclonal antibodies were followed by fixation with 4% paraformaldehyde to prevent subsequent migration of the two secondary antibodies.
This technique could only be used safely for antibody combinations that were at least in part exclusive in their staining patterns and could therefore be checked for “cross-binding” or other unspecific staining not mediated by the binding of the specific primary antibody to its specific antigen.
Secondary antibodies used were: (1) Alexa 594 conjugated goat anti-mouse IgG (1:200), (2) Alexa 488 conjugated goat anti-mouse IgG (1:200) and (3) Alexa 488 conjugated goat anti-rabbit IgG (1:400; all Molecular Probes, Cambridge BioScience, Cambridge, UK). The secondary antibodies were diluted in PBS and applied for 1 h at room temperature. Nuclei were stained by 2 min incubation in a solution of bisbenzimide (0.5 g/ml; Sigma-Aldrich) in PBS at room temperature prior to the final wash. The sections were finally mounted in Fluoromount-G (EMS, Science Services, London, UK). All quantitative analysis was carried out with a Zeiss Axiophot microscope equipped with a b/w CCD camera (Carl Zeiss, Welwyn Garden City, UK) and using Metamorph software (Universal Imaging Co., Marlow, UK). Additional images were made using a Leica TCS SP1 confocal microscope with the respective software package (Leica Microsystems, Milton Keynes, UK).
To facilitate the re-identification of the individual satellite cells for the quantitative analysis, one cryosection was stained with anti-Pax7 and anti-Mcad while its two adjacent sections (i.e. the ones cut directly before and after) were stained with anti-Mcad and anti-laminin. Further sections were used for the investigation of the MRF in relation to anti-Pax7. In each case at least every second section was stained with either anti-laminin or anti-collagen type IV to ensure correct morphological correlation.
Tissue culture
Foetal human limb muscle was provided by the MRC tissue bank in compliance with the government approved recommendations of the Polkinghorne Report (1989) and with permission from the research ethics committee of the Hammersmith, Queen Charlotte’s, Chelsea and Acton Hospitals, London. Explant cultures were set up by enzymatic disaggregation technique, similar to previous descriptions (Beauchamp et al. 1999). In short, the muscle was removed and finely minced in 25 µg/ml fungizone (Invitrogen, Paisley, UK) and 2% penicillin/streptomycin (Sigma-Aldrich) in PBS, washed in PBS and then incubated in 0.1% type I collagenase (Sigma-Aldrich), 0.1% bovine serum albumin (Sigma-Aldrich), 2% l-glutamine (Sigma-Aldrich), 1% penicillin/streptomycin, and 8% trypsin 10X (Sigma-Aldrich) in Ham’s F10 nutrient mixture (Gibco, Invitrogen, Paisley, UK) at 37°C for 10 min. After vigorous shaking, the supernatant was removed and mixed with an equal volume inhibition growth medium [Ham’s F10 with 20% foetal bovine serum (FBS; Invitrogen), 2% l-glutamine, 1% penicillin/streptomycin]. This procedure was repeated with the remaining undigested muscle parts and the suspensions were pooled, triturated with a pipette, filtered, centrifuged and finally the pellet dispersed in inhibition growth medium. The cells were then counted and seeded at 104/cm2 in a sterile flask coated with 0.1% gelatine (Sigma-Aldrich) in sterile water. After several changes of medium the cells were trypsinised, counted and, provided that immunostaining of a sample indicated a fraction of desmin positive cells above 70%, frozen down or directly seeded again for experiments. When the cells had reached approximately 70–80% confluence in inhibition growth medium, the medium was changed to Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Invitrogen, Paisley, UK) with 4% FBS, 2% l-glutamine, and 1% penicillin/streptomycin to induce differentiation.
For histological analysis, the cells were seeded in 10-cm culture dishes into which autoclaved 22×22-mm glass coverslips had been placed. At the different time points a dish was washed twice with PBS and the coverslips were removed and fixed (see above).
RNA for RT-PCR was extracted from a 175-cm2 culture flasks content of cells for each time point using Trizol reagent (Gibco Brl, Invitrogen), following the manufacturer’s protocol.
The cells used for this study were obtained from the limb muscles of a 14-week-old foetus (Fig. 5).
Cell counts
Images of five random camera fields per coverslip/timepoint were acquired at a primary magnification of ×20 and the stained nuclei counted using the described staining methods and microscope/software set-up. A nucleus was only counted as positive for an immunostaining when the shape of the nucleus as indicated by bisbenzimide could clearly be distinguished from the background. To assess the number of Pax7-positive cells in correlation not only to differentiation time of the culture but also to the ongoing proliferative activity in that culture, a triple staining with anti-Pax7, anti-PNCA-FITC and bisbenzimide was used on cultures from differentiation days 0 (before medium switch), 2, 5, 8, and 10. The counts for Pax7 and for PCNA, respectively, were divided by the total nuclei (bisbenzimide) count to represent the fraction of cells positive for Pax7 and PCNA, respectively. The counts for the five different time points were then compared by one-way ANOVA.
RT-PCR
RNA samples from cultures before and 2, 4, 7, 10, and 12 days after induction of differentiation were tested for the presence of Pax7 mRNA by RT-PCR using the primer pair (5’-GCAAGCAGCGACGCAGTCG-3’) and (5’-GAGAAGTCAGCCTGTGGCTGCG-3’) with the Quiagen One-step RT-PCR kit (Quiagen, Crawley, UK) according to the manufacturer’s instructions. RNA quantity and quality were assessed by spectrophotometry and gel electrophoresis. For each reaction, 1.5 µg RNA was used and control RT-PCR for GAPDH was carried out using the primer pair (5’-TTCATTGACCTCAACTACATG-3’) and (5’-GTGGCAGTGATGGCATGGAC-3’) with the same kit. Expected product sizes were 571 base pairs for Pax7 and 443 base pairs for GAPDH, respectively, according to GeneBank data (X96743 and M17851, respectively). To confirm the sequence of our Pax7 product, one sample of fresh RT-PCR product was cloned and sequenced using the TOPO TA cloning kit (Invitrogen).
Results
Antibody reactivity
In both normal and pathological muscle, anti-Mcad reacted with the areas of contact between satellite cells and their muscle fibres (Fig. 1a, d). In pathological samples, however, it also showed reactivity with the membranes of myotubes and regenerated myofibres and with occasional mononuclear cells outside the fibres’ basement membranes, presumably myoblasts or myocytes. In samples with extensive regeneration there also was a considerably weaker reactivity to be detected on the membranes of some myofibres with diameters too great to be considered freshly regenerated.
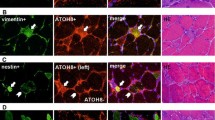
Adjacent sections (a–d and e–h) of two satellite cells facing each other, one Pax7 positive, one negative. a, e Anti-Mcad, marking the interface between satellite cells and myofibres (arrowheads); b anti-Pax7, reactive with one of the satellite cell nuclei; c, g bisbenzimide indicating the site of the nuclei; f anti-laminin staining giving the location of the basal laminae of myofibres and vessels. Picture taken from the biceps brachii muscle of a case of Becker muscular dystrophy, as listed in Table 1. Primary magnification ×63. Scale bar 20 µm
Anti-Pax7 reactivity was restricted to a small population of nuclei, mostly corresponding to satellite cells (Fig. 1b; see below). No extranuclear staining patterns were observed.
Specific reactivity of either antibody with vessels, nerves or connective tissue was not observed.
Satellite cells
Using double immunostainings for anti-Mcad/ anti-laminin and anti-Mcad/ anti-Pax7 with additional nuclear staining (bisbenzimide) of adjacent cryosections, altogether 1,078 satellite cell nuclei in 11 muscles were identified by Mcad immunoreactivity, cell morphology and histological location. The counts per biopsy sample section ranged from 18 to 225. Of these, 49 did not show anti-Pax7 reactivity (Fig. 1b), ranging from 2 to 10 per section (evaluated muscle section areas between 0.17 and 2.73 mm2). The percentage of nuclei of Mcad positive satellite cells that were negative for Pax7 ranged from 1.6% to 15.2% (average 6.3%; median 5.0%). The counts per biopsy together with the biopsy details are listed in Table 1.
Regenerating/ degenerating fibres
We were not able to demonstrate a single Pax7-positive nucleus in myotubes newly formed in regeneration, as identified by their small, round profiles in anti-Mcad staining or by staining for neonatal myosin heavy chain (nMHC) on serial sections (not shown). While this differs from observations in regenerated murine muscle (Seale et al. 2000), it is in line with our observations in tissue culture (see below). This picture was further complicated by the presence of a small population of Pax7-positive nuclei that did not correspond to the criteria of satellite cells. These nuclei were found in muscle fibres showing morphological signs of degenerative changes, i.e. deformed profiles with non-homogeneous sarcoplasm and some non-peripheral nuclei. Often they had the elongated, angular shape characteristic of partial atrophy. Within these fibre profiles, that were always found surrounded by an intact basal lamina, usually several nuclei with anti-Pax7 reactivity of varying intensity were found (Fig. 2e–h). Re-identification of such fibres on serial sections double-stained with anti-laminin and anti-Mcad, however, revealed the location of these nuclei in several compartments separated by M-cad positive membranes within the basal lamina (Fig. 2a–d). On serial sections stained with anti-nMHC, some areas within the basal lamina profile were immunoreactive, but the areas corresponding to the mentioned compartments showed no reactivity (Fig. 2i–l). Further serial sections also revealed some reactivity in these Pax7-positive nuclei that occupy those Mcad positive, nMHC negative compartments with anti-Myf-5 and anti-MyoD1, but little to none with anti-myogenin (not shown).
Serial sections of a muscle biopsy from the biceps brachii muscle of a patient suffering from Becker muscular dystrophy, as listed in Table 1. a, e Anti-Mcad staining indicates the membranes inside the compartmented (one of the compartments is marked by an asterisk in a) profile of a myofibre with an angular shape as well as two satellite cells on neighbouring fibres (arrowheads). Anti-laminin (b) and anti-collagen type IV (i) demarcate the basal laminae of myofibres and vessels. Bisbenzimide indicates the site of the nuclei in c, g and k. Note the anti-Pax7 reactivity (f) of the nuclei (g) inside the Mcad positive compartments (e) of the angular fibre profile and the nucleus of one of the satellite cells. Anti-neonatal myosin heavy chain (j) reacts with the sarcoplasm of the angular fibre, but spares areas (e.g. the one with the asterisk in j) corresponding to some of the compartments lined out by anti-Mcad in a and e. The first two sections (a–d and e–h) are directly adjacent sections, while the third (i–l) is one of the further sections in the series. Primary magnification ×40. Scale bar 20 µm
Muscle spindles
In contrast to earlier reports based on in situ hybridisation in mouse muscle (Rodger et al. 1999), we were not able to detect any anti-Pax7 reactivity in the capsules of human muscle spindles (Fig. 3). Of the satellite cells identified by anti-Mcad/anti-laminin on the intrafusal fibres, all were positive for Pax7. However, muscle spindles are rarely found in biopsies of adult muscle. This biases our data on spindles first to samples from a younger age group and second to biopsies from clinically apparent neuromuscular conditions.
A muscle spindle in the muscle of a 2-month-old girl not listed in Table 1 as mentioned in “Materials and methods”. a Anti-collagen type IV marking the basal lamina structures and the spindle’s capsule. b Anti-Pax7 staining the nuclei of satellite cells on both intrafusal and extrafusal muscle fibres, but no structures of the spindle’s capsule. c Bisbenzimide indicating the location of the nuclei. Primary magnification ×63. Scale bar 20 µm
Tissue culture
In myogenic cultures derived from human foetal skeletal muscle only a minority of cells showed immunoreactivity with anti-Pax7 prior to the induction of differentiation. However, the proportion of positive nuclei and the intensity of their reactivity increased with days in differentiation medium (Fig. 4). Triple stainings with anti-Pax7, anti-PCNA (FITC-conjugate) and bisbenzimide were analysed before medium change (day 0) and 2, 5, 8, and 10 days after the switch to differentiation medium. Counts of five random fields per 22×22 mm coverslip revealed a significantly greater proportion of nuclei positive for Pax7 at day 10 than at day 0, day 2 and day 5 (significant differences between the groups in one-way ANOVA, P=0.005; day 10 significantly higher than days 0, 2, 5; P values: 0.008, 0.012, 0.017, respectively, in a Tukey test pairwise comparison). The proportion of nuclei positive for PCNA, in contrast, was significantly reduced during differentiation (significant differences between the groups in one-way ANOVA, P<0.001; day 0 significantly higher than days 2, 5, 8, 10; P values: 0.007, 0.006, <0.001, <0.001, respectively, in a Tukey test pairwise comparison). Nuclei intensely positive for PCNA showed generally little or no reactivity for anti-Pax7. However, some co-expression of low level PCNA with Pax7 was observed (not shown). It is also noteworthy that staining with anti-desmin and anti-PCNA in parallel demonstrated that the cells with high level PCNA expression in the later days of differentiation were predominantly desmin negative (data not shown). The implication of continuing proliferation of non-myogenic cells in cultures under conditions aiming for the differentiation of myogenic cells was supported by a gradual reduction in the fraction of nuclei that could be attributed to muscle cells in the anti-desmin staining with time in differentiation medium. Of this fraction before the induction of differentiation, 99.2% were found in mononuclear cells, while 0.8% belonged to cells with more than one but less than four nuclei, whereas 72.9%, 67.5%, 64.0% and 50.1% were attributed to mononuclear cells at days 2, 5, 8 and 10, respectively. The percentage of nuclei located in polynucleate cells with less than four nuclei was 20% at day 2, and 24.1%, 22.0% and 24.6% at days 5, 8 and 10, respectively. Myotubes with more than three nuclei were not present before the induction of differentiation. The percentage of their nuclei increased from 7.1% at day 2 to 8.4%, 14.0% and 25.3% at days 5, 8 and 10, respectively. Regarding these figures it is important to note that, as these cells grew very dense with time and in some areas in several layers, correct attribution of individual nuclei to cell shapes became increasingly difficult. Double staining with anti-desmin and anti-Pax7 in these cultures gave no indication of a Pax7-positive, desmin-negative population (data not shown).
Myf-5 and MyoD1, present in the majority of cells before the induction of differentiation and persisting well into the early days of differentiation time, were regularly found co-expressed with Pax7 in mononuclear cells at the differentiation stages (Fig. 5a–f). A small proportion of Pax7-positive cells was negative for these markers and vice versa. In contrast, myogenin and Pax7 were almost exclusive (Fig. 5g–i). The rare cases of co-expression of low level Pax7 with myogenin were usually mononuclear myocytes and few early myotubes (up to three nuclei). Pax7 was not observed in more mature, polynucleate myotubes.
Tissue culture cells. a–c Human foetal muscle cells at differentiation day 5 stained with anti-Myf5 (a), anti-Pax7 (b) and bisbenzimide (c). d–f Differentiation day 4 showing reactivity with anti-MyoD1 (d), anti-Pax7 (e), and bisbenzimide (f). At differentiation day 6 marked for myogeninin (g), Pax7 (h) and bisbenzimide (i). Note the multinucleated myotube in g–i. These cells were obtained from the limb muscles of a 14-week-old foetus. Primary magnification ×40. Scale bar 20 µm
RT-PCR
As Pax7 mRNA has been identified in proliferating myoblasts (Seale et al. 2000), we investigated total RNA extractions of our myogenic cultures to correlate it to the presence of immunoreactive protein. The RT-PCR indeed indicated the presence of Pax7 mRNA from before the start of differentiation throughout the differentiation stages. Some of the levels in differentiating cultures were higher than in the proliferation stage (day 7, day 10; Fig. 6), but others were found to be lower (day 4, day 12; Fig. 6). Sequencing of cloned RT-PCR product derived from differentiation day 7 total RNA verified it as the target Pax7 sequence.
Gel electrophoresis of RT-PCR products for Pax7 (upper part) and GAPDH (lower part) from total RNA of human foetal muscle cell cultures prior to and at different times after the change to differentiation medium. On overexposed pictures, two very faint Pax7 bands could be detected at day 4 and day 12, too
Discussion
In human muscle, the presence of satellite cell nuclei negative for Pax7 protein, as well as that of Pax7 protein positive nuclei in myofibres, argues strongly against the use of anti-Pax7 alone as a satellite cell “marker” in man. However, in the absence of an absolute criterion for the definition of all satellite cells across species or in pathological conditions, ex cathedra pronouncements should be avoided. Certainly, in our study anti-Mcad identified more cells in satellite cell position than anti-Pax7. This included all Pax7-positive nuclei present, except for those few inside fibre profiles. While our counts indicate the number of satellite cells that would not have been detected by use of anti-Pax7 as a marker alone, this may not be the precise number of Pax7-negative satellite cells. Due to the technical limitations of serial cryosectioning and staining, it seems possible that a section occasionally may contain a tiny slice of a nucleus with sufficient DNA for a signal with bisbenzimide but with insufficient Pax7 protein for detectable immunolabelling. However, this is unlikely to be a serious problem since even very small Pax7-positive nuclear profiles were found. Moreover, some large Pax7-negative nuclear profiles in the Mcad-positive population of satellite cells are clear evidence of a genuine Pax7-negative population.
The detection of Pax7-positive nuclei in myofibre profiles of pathological muscle has two alternative explanations: (a) non-functional re-expression of “early” transcription factors, or (b) regenerative cells in a transition stage. The former possibility cannot be completely ruled out. The co-expression of the early myogenic regulatory factors (MRF) Myf5 and MyoD1 found in some of these nuclei is not a differentiating criterion, as such re-expression in fibre myonuclei has been reported, e.g. in denervation (Weis 1994). However, the second explanation seems the more likely in the majority of cases, because of the presence of anti-Mcad-immunoreactive membranes segregating the individual compartments containing most of these Pax7-positive nuclei from the rest of the fibre profile area as delimited by its basal lamina. The morphological and immunohistochemical data collected here suggest that these are pre-fusion myoregenerative cells engaged in repair of damaged muscle fibres. Interestingly, these cells closely resemble the majority of the Pax7-positive population in cell culture. Combining the data sets, both populations seem to be mononuclear cells beyond the proliferation stage but prior to final differentiation and fusion. The morphological evaluation is paralleled in culture by an inverse relationship of Pax7 and both PCNA, which is maximal at S phase, and myogenin, which is typically associated with the later stages of differentiation. The co-expression in differentiating cultures of Pax7 with Myf5 and MyoD1 together with the increase of the Pax7-positive population with progressing differentiation time, likewise agree with this view. From this point of view, all cells with Pax7-positive nuclei in our study: satellite cells, regenerative cells in fibre profiles and the cells we observed in culture, seem to be in an intermediate state between proliferation and final differentiation, although engaged in different processes.
The differences between Pax7 protein expression and the presence of Pax7 mRNA as detected by immunostaining and RT-PCR, respectively, and the varying amounts of RT-PCR product in our myogenic cultures might arise from a number of factors. One-step RT-PCR is a very sensitive technique and cannot be considered quantitative, especially not when total RNA samples from different differentiation stages are concerned. There also is a wide range of differentiation stages in such cultures, detectable even on a 22×22 coverslip (see Fig. 4). Thus, disproportionate representations seem a likely explanation. The aim of our RT-PCR was to test for the presence of Pax7 mRNA in proliferating and differentiating cultures only as our tissue culture data differs from descriptions in the mouse (Seale et al. 2000). Despite this, the regulation of Pax7 mRNA translation and its relation to the early MRF appear to be important issues for further investigation.
The notion of Pax7-negative satellite cells adds to the increasing evidence of the heterogeneity of satellite cells (Rantanen et al. 1995; Molnar et al. 1996; Lagord et al. 1998; Beauchamp et al. 1999, 2000). Different populations of satellite cells have been a recurring theme even before the advent of the idea that cells from outside the myofibres’ basal laminae may join the myoregenerative population, by “transdifferentiation” or by recruitment of a stem cell not previously committed to the muscle lineage (LaBarge and Blau 2002). In view of such potential diversity, it would not be surprising that a minority of satellite cells in our investigation do not have levels of Pax7 protein detectable by immunohistochemistry. Their paired box binding sites might be occupied by another member of the Pax family, fulfilling whichever functions in transcriptional regulation to lead to their specification as satellite cells. Pax3 seems an obvious candidate bearing in mind its presence in migratory muscle precursor cells during development and its high degree of homology with Pax7. This idea is supported by the detection of Pax3 expression in satellite cells of murine gracilis muscles (Buckingham et al. 2003). Lack of a specific anti-Pax3 antibody for the human prevented further investigation of this possibility.
Our tissue culture data suggests the alternative that these might be cells in transit from the quiescent satellite state that requires Pax7, to the active myoblast stage that, in man, apparently does not. Repression of Pax7 at the right time point has been shown to be essential for some neuronal precursors to enter the road to differentiation as a motor neuron (Ericson et al. 1996). This later theory is not supported by our data from regenerating muscles, indicating no shift towards Pax7-negative, Mcad-positive satellite cells, although our small sample size may lack the sensitivity to detect this change.
It is important to note that this discussion conforms to a tacit assumption made in previous ideas on satellite cell heterogeneity. This is the use of a model, adapted from developmental biology, of a unidirectional hierarchy of step-by-step myogenesis in which the satellite cell is seen as a terminal or pre-terminal progenitor of the committed myoblast. This analogy is bolstered by the involvement of “developmental genes” such as Pax7. Yet the satellite cell’s function is not de novo development of an organ, but growth and repair of the existing muscle: a maintenance that seems to involve the segregation of new satellite cells, likely by asymmetrical division (Conboy and Rando 2002). This differs radically from muscle development, involving as it does the transition from quiescence to proliferation and “back” to quiescence to preserve a tissue status quo. We have therefore to consider the aptness of this developmental model and consider whether the heterogeneous populations of satellite cells might reflect their dynamism rather than their position in a lineage such that the differences we describe could rather be the aspects of a cycle of functional activity. It would be fitting that these manifest in the expression pattern of a transcription factor such as Pax7 rather than of an adhesion molecule with a slow turnover such as Mcad. Further study and manipulation of Pax7 should provide answers to these questions.
References
Beauchamp JR, Morgan JE, Pagel, Partridge TA (1999) Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 144:1113–1122
Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit P (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1234
Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F (2003) The formation of skeletal muscle: from somite to limb. J Anat 202:59–68
Conboy IM, Rando TA (2002) The regulation of notch signalling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3:397–409
Donalies M, Cramer M, Ringwald M, Starzinski-Powitz A (1991) Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation in skeletal muscle cells. Proc Natl Acad Sci U S A 88:8024–8028
Epstein JA, Shapiro DN, Cheng J, Lam PYP, Mass RL (1996) Pax3 modulates expression of the c-met receptor during limb muscle development. Proc Natl Acad Sci U S A 93:4212–4218
Ericson J, Morton S, Kawakami A, Roelink H, Jessel TM (1996) Two critical periods of sonic hedgehog signaling required for the specification of motor neuron identity. Cell 87:661–673
Heslop L, Beauchamp JR, Tajbakhsh S, Buckingham ME, Partridge TA, Zammit PS (2001) Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther 8:778–783
Holst BD, Gommer RS, Wood IC, Edelman GM, Jones FS (1994) Binding and activation of the promoter for the neural cell adhesion molecule by Pax-8. J Biol Chem 269:2224–2225
Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199:326–337
LaBarge MA, Blau HM (2002) Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111:589–601
Lagord C, Soulet L, Bonavaud S, Bassaglia Y, Rey C, Barlovatz-Meimon G, Gautron J, Martelly I (1998) Differential myogenicity of satellite cells isolated from extensor digitorum longus (EDL) and soleus rat muscles revealed in vitro. Cell Tissue Res 291:455–468
Mansouri A, Hallonet M, Gruss P (1996) Pax genes and their role in cell differentiation and development. Curr Opin Cell Biol 8:851–857
Molnar G, Ho ML, Schroedl NA (1996) Evidence for multiple satellite cell populations and a non-myogenic cell type that is regulated differently in regenerating and growing skeletal muscle. Tissue Cell 28:547–556
Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H (1995) Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72:341–347
Rodger J, Ziman MR, Papadimitriou JM, Kay PH (1999) Pax7 is expressed in the capsules surrounding adult mouse neuromuscular spindles. Biochem Cell Biol 77:153–156
Rosenblatt JD, Cullen MJ, Irintchev A, Wernig A (1999) M-cadherin is a reliable molecular marker of satellite cells in mouse skeletal muscle. Eur J Physiol 437:R145 (abstract)
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki ME (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Watkins SC, Cullen MJ (1988) A quantitative study of myonuclear and satellite cell nuclear size in Duchenne’s muscular dystrophy, polymyositis and normal human skeletal muscle. Anat Rec 222:6–11
Weis J (1994) Fos, Jun, Myo D1 and myogenin proteins are increased in skeletal muscle fibre nuclei after denervation. Acta Neuropathol 87:63–70
Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA (2002) Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibres. Exp Cell Res 281:39–49
Acknowledgement
Foetal tissue was provided by the MRC tissue bank in compliance with the government approved recommendations of the Polkinghorne Report (1989) and with permission from the Research Ethics Committee of the Hammersmith, Queen Charlotte’s, Chelsea and Acton Hospitals, London. We wish to thank Prof. Atsushi Kawakami for the development of the anti-Pax7 hybridoma and for his friendly advice. Mrs. Karin Kappes-Horn provided invaluable organisational help again. Prof. Kawakami’s Pax7 hybridoma was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. F. Reimann was supported by a grant from the Deutsche Forschungsgemeinschaft (Re 1547/1–1). Further support came from the Muscular Dystrophy Association (Prof. Partridge)
Rights and permissions
About this article
Cite this article
Reimann, J., Brimah, K., Schröder, R. et al. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res 315, 233–242 (2004). https://doi.org/10.1007/s00441-003-0833-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0833-y