Abstract
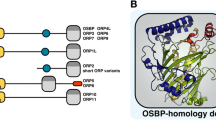
The human OSBP related protein (ORP) family consists of 12 members, which can be divided into six subfamilies based on the genomic organization and amino acid homology. Here we performed basic characterization of subfamily III, which consists of three members: ORP3, ORP6, and ORP7. According to cDNA hybridization, the three genes are expressed in a tissue-specific manner. While ORP3 mRNA is most abundant in kidney, lymph nodes, and thymus, ORP6 shows highest expression in brain and skeletal muscle, and ORP7 in the gastrointestinal tract. Using monospecific peptide antibodies, we confirmed the presence of the three proteins in human and mouse tissues. ORP6 gene expression was induced upon differentiation of F9 embryonic carcinoma cells into parietal endoderm, while ORP3 and ORP7 mRNA levels were unchanged. In the F9 cells, endogenous ORP6 associated predominantly with the nuclear envelope. When expressed from the cDNA in cultured cells, the three proteins were distributed between the cytosol and endoplasmic reticulum (ER) membranes, with a minor portion found at the plasma membrane. Experiments with truncated constructs showed that the N-terminal portion of the proteins, containing a pleckstrin homology (PH) domain, has markedly strong plasma membrane targeting specificity, while the C-terminal half remains largely cytosolic. The expression data demonstrates that ORP3, -6, and -7 are not merely redundant gene products but show marked quantitative differences in tissue expression, suggesting tissue-specific aspects in their function. The dual targeting of the proteins indicates a putative role in communication between the ER and the plasma membrane.












Similar content being viewed by others
References
Anniss AM, Apostolopoulos J, Dworkin S, Purton LE, Sparrow RL (2002) An oxysterol-binding protein family identified in the mouse. DNA Cell Biol 21:571–580
Beh CT, Cool L, Phillips J, Rine J (2001) Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 157:1117–1140
Beséme F, Astruc ME, Defay R, Descomps B, Crastes de Paulet A (1986) Characterization of oxysterol-binding protein in rat embryo fibroblasts and variations as a function of the cell cycle. Biochim Biophys Acta 886:96–108
Collier FM, Grecorio-King CC, Apostolopoulos J, Walder K, Kirkland MA (2003) ORP3 splice variants and their expression in human tissues and hematopoietic cells. DNA Cell Biol 22:1–9
Covell DC, Wallqvist A, Rabow AA, Thanki N (2003) Molecular classification of cancer: Unsupervised selforganizing map analysis of gene expression microarray data. Mol Cancer Ther 2:317–332
Daum G, et al. (1999) Systematic analysis of yeast strains with possible defect in lipid metabolism. Yeast 15:601–614
Dawson PA, Ridgway ND, Slaughter CA, Brown MS, Goldstein JL (1989) cDNA cloning and expression of oxysterol-binding protein, an oligomer with a potential leucine zipper. J Biol Chem 264:16798–16803
Defay RE, Astruc ME, Roussillon S, Descomps B, Crastes de Paulet A (1982) A specific hydroxysterol binding protein in human lymphocyte cytosol. Biochimie 64:331–339
Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA (1996) Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J 15:6447–6459
Fournier MV, Guimaraes FC, Paschoal MEM, Ronco LV, Carvalho MGC, Pardee AB (1999) Identification of a gene encoding a human oxysterol-binding protein-homologue: a potential general molecular marker for blood dissemination of solid tumors. Cancer Res 59:3748–3753
Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJM, Desjardins M (2002) Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110:119–131
Gregorio-King CC, Collier GR, McMillan JS, Waugh CM, McLeod JL, Collier FM, Kirkland MA (2001) ORP-3, a human oxysterol-binding protein gene differentially expressed in hematopoietic cells. Blood 98:2279–2281
Grether ME, Herskowitz I (1999) Genetic and biochemical characterization of the yeast Spo12 protein. Mol Biol Cell 10:3689–3703
Hanley K, Ng DC, He SS, Lau P, Min K, Elias PM, Bikle DD, Mangelsdorf DJ, Williams ML, Feingold KR (2000) Oxysterols induce differentiation in human keratinocytes and increase Ap-1-dependent involucrin transcription. J Invest Dermatol 114:545–553
Harris TM, Childs G (2002) Global gene expression patterns during differentiation of F9 embryonal carcinoma cells into parietal endoderm. Funct Integr Genomics 2:105–119
Hayden JM, Brachova L, Higgins K, Obermiller L, Sevanian A, Khandrika S, Reaven PD (2002) Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J Lipid Res 43:26–35
Ho Y, et al. (2002) Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180–183
Iacobuzio-Donahue CA, et al. (2002) Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol 160:1239–1249
Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y (2001) A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci U S A 98:4569–4574
Jaworski CJ, Moreira E, Li A, Lee R, Rodriguez IR (2001) A family of 12 human genes containing oxysterol-binding domains. Genomics 78:185–196
Jelinek DF, Tschymber RC, Stolovitsky GA, Iturria SJ, Tu Y, Lepre J, Shah N, Kay NE (2003) Identification of a global gene expression signature of B-chronic lymphocytic leukaemia. Mol Cancer Res 1:346–361
Jiang B, Brown JL, Sheraton J, Fortin N, Bussey H (1994) A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein. Yeast 10:341–353
Johansson M, Bocher V, Lehto M, Chinetti G, Kuismanen E, Ehnholm C, Staels B, Olkkonen VM (2003) The two variants of oxysterol binding protein-related protein-1 display different tissue expression patterns, have different intracellular localization, and are functionally distinct. Mol Biol Cell 14:903–915
Kandutsch AA, Shown EP (1981) Assay of oxysterol-binding protein in a mouse fibroblast, cell free system. J Biol Chem 256:13068–13073
Kandutsch AA, Taylor FR, Shown EP (1984) Different forms of oxysterol-binding protein. J Biol Chem 259:12388–12397
Lagace TA, Byers DM, Cook HW, Ridgway ND (1997) Altered regulation of cholesterol and cholesteryl ester synthesis in Chinese-hamster ovary cells overexpressing the oxysterol-binding protein is dependent on the pleckstrin homology domain. Biochem J 326:205–213
Lagace TA, Byers DM, Cook HW, Ridgway ND (1999) Chinese hamster ovary cells overexpressing the oxysterol binding protein (OSBP) display enhanced synthesis of sphingomyelin in response to 25-hydroxycholesterol. J Lipid Res 40:109–116
Laitinen S, Olkkonen VM, Ehnholm C, Ikonen E (1999) Family of human oxysterol binding protein (OSBP) homologues: a novel member implicated in brain sterol metabolism. J Lipid Res 40:2204–2211
Laitinen S, Lehto M, Lehtonen S, Hyvärinen K, Heino S, Lehtonen E, Ehnholm C, Ikonen E, Olkkonen VM (2002) ORP2, a homolog of oxysterol binding protein, regulates cellular cholesterol metabolism. J Lipid Res 43:245–255
Lapteva N, Nieda M, Ando Y, Nicol A, Ide K, Yamaura A, Hatta-Ohashi Y, Egawa K, Juji T, Tokunaga K (2001) Gene expression analysis in human monocytes, monocyte-derived dendritic cells, and α-galactosylceramide-pulsed monocyte-derived dendritic cells. Biochem Biophys Res Comm 289:531–538
Lehto M, Olkkonen VM (2003) The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim Biophys Acta 1631:1–11
Lehto M, Laitinen S, Chinetti G, Johansson M, Ehnholm C, Staels B, Ikonen E, Olkkonen VM (2001) The OSBP-related protein family in humans. J Lipid Res 42:1203–1213
Lemmon MA, Ferguson KM (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350:1–18
Levanon D, Hsieh C-L, Francke U, Dawson PA, Ridgway ND, Brown MS, Goldstein JL (1990) cDNA cloning of human oxysterol-binding protein and localization of the gene to human chromosome 11 and mouse chromosome 19. Genomics 7:65–74
Levine TP, Munro S (1998) The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr Biol 8:729–739
Levine TP, Munro S (2001) Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol Biol Cell 12:1633–1644
Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA (2002) Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol 157:63–77
Maffucci T, Falasca M (2001) Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide-protein co-operative mechanism. FEBS Lett 506:173–179
Mohammadi A, Perry RJ, Storey MK, Cook HW, Byers DM, Ridgway ND (2001) Golgi localization and phosphorylation of oxysterol binding protein in Niemann-Pick C and U18666A-treated cells. J Lipid Res 42:1062–1071
Panini SR, Sinensky MS (2001) Mechanisms of oxysterol-induced apoptosis. Curr Opin Lipidol 12:529–533
Park Y-U, Hwang O, Kim J (2002) Two-hybrid cloning and characterization of OSH3, a yeast oxysterol-binding protein homolog. Biochem Biophys Res Comm 293:733–740
Patterson RL, Rossum DB, Gill DL (1999) Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell 98:487–499
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acid Res 29:2002–2007
Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G (2001) A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem 268:2351–2361
Ridgway ND, Dawson PA, Ho YK, Brown MS, Goldstein JL (1992) Translocation of oxysterol binding protein to Golgi triggered by ligand binding. J Cell Biol 116:307–319
Ridgway ND, Lagace TA, Cook HW, Byers DM (1998) Differential effects of sphingomyelin hydrolysis and cholesterol transport on oxysterol-binding protein phosphorylation and Golgi localization. J Biol Chem 273:31621–31628
Rizzino A (2002) Embryonic stem cells provide a powerful and versatile model system. Vitam Horm 64:1–42
Ross SE, et al. (2002) Microarray analyses during adipogenesis: Understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol Cell Biol 22:5989–5999
Schroepfer GJ (2000) Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev 80:361–554
Silva NH, Pimenta G, Pulcheri WA, Fournier MV, Spector N, Carvalho MDC (2001) Detection of messenger RNA in leucocytes or plasma of patients with chronic myeloid leukemia. Oncol Rep 8:693–696
Simonsen A, Bremnes B, Ronning E, Aasland R, Stenmark H (1998) Syntaxin-16, a putative Golgi t-snare. Eur J Cell Biol 75:223–231
Strickland S, Mahdavi V (1978) The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 15:393–403
Strickland S, Smith KK, Marotti KR (1980) Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell 21:347–355
Tanaka TS, Kunath T, Kimber WL, Jaradat SA, Stagg CA, Usuda M, Yokota T, Niwa H, Rossant J, Ko MSH (2002) Gene expression profiling of embryo-derived stem cells reveals candidate genes associated with pluripotency and lineage specificity. Genome Res 12:1921–1928
Taylor FR, Saucier SE, Shown EP, Parish EJ, Kandutsch AA (1984) Correlation between oxysterol binding to a cytosolic binding protein and potency in the repression of hydroxymethylglutaryl coenzyme A reductase. J Biol Chem 259:12382–12387
Whitfield ML, et al. (2002) Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13:1977–2000
Wyles JP, McMaster CR, Ridgway ND (2002) Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem 277:29908–29918
Xu Y, Liu Y, Ridgway ND, McMaster CR (2001) Novel members of the human oxysterol-binding protein family bind phospholipids and regulate vesicle transport. J Biol Chem 276:18407–18414
Yamazaki K, Aso T, Ohnishi Y, Ohno M, Tamura K, Shuin T, Kitajima S, Nakabeppu Y (2003) Mammalian Elongin A is not essential for cell viability but required for proper cell-cycle progression with limited alteration of gene expression. J Biol Chem 278:13585–13589
Acknowledgements
We are grateful to Ulla Kiiski, Tuulikki Leinonen, Seija Puomilahti, and Pirjo Ranta for expert technical assistance, to Dr. Giulia Chinetti (Pasteur Institute, Lille, France) for the monocyte and macrophage protein specimens, and to Dr. Ilkka Reima for critically reading the manuscript. The KIAA0704 cDNA was kindly provided by Dr. Takahiro Nagase (Kazusa DNA Research Institute, Kisarazu, Chiba, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Clinical Research Fund of Helsinki University Central Hospital (J.T.), the Academy of Finland (grant 51883 to M.L.; grants 49987, 50641, and 54301 to V.M.O.), the Sigrid Juselius Foundation, and the Finnish Cultural Foundation
Rights and permissions
About this article
Cite this article
Lehto, M., Tienari, J., Lehtonen, S. et al. Subfamily III of mammalian oxysterol-binding protein (OSBP) homologues: the expression and intracellular localization of ORP3, ORP6, and ORP7. Cell Tissue Res 315, 39–57 (2004). https://doi.org/10.1007/s00441-003-0817-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0817-y