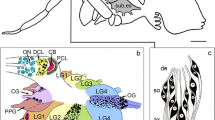
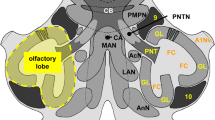
Abstract
Antisera against corazonin were used to investigate distribution of immunoreactive cells in the central nervous system (CNS) of representatives of six insect orders: Ctenolepisma lineata (Zygentoma), Locusta migratoria (Orthoptera), Oxya yezoensis (Orthoptera), Gryllus bimaculatus (Orthoptera), Pyrrhocoris apterus (Hemiptera), Arge nigrinodosa (Hymenoptera), Athalia rosae (Hymenoptera), Bombyx mori (Lepidoptera) and Anomala cuprea (Coleoptera). Corazonin-like immunoreactive (CLI) cells were detected in the brain and ventral ganglia of all insects studied except for the albino strain of L. migratoria and the beetle A. cuprea. Implantation of the brain or different ganglia from insects with detected immunoreactivity induced dark coloration in the albino locust, providing further evidence for the presence of authentic corazonins [His7- and Arg7-isoforms] in these insects. The protocerebral lateral neurosecretory cells projecting into the ipsilateral retrocerebral neurohemal organs and bilateral longitudinal tracts extending and branching throughout the entire CNS seem to be a well-conserved part of the corazonin system in insects. The bilateral longitudinal tracts were formed by species-specific numbers of bilateral interneurons segmentally distributed in the ventral ganglia. Additional immunoreactive somata, mostly interneurons, were detected in the CNS of various insects. The distribution of corazonin in the cephalic neurosecretory system and in the bilateral interneurons suggests that corazonin acts as a hormone as well as a neurotransmitter or a neuromodulator. An ancient origin of corazonin is suggested by the presence of a corazonin-like substance in the primitive insect, C. lineata. These results support previous findings on the common occurrence of corazonin among insects, except for the albino strain of L. migratoria and the Coleoptera.







Similar content being viewed by others
Abbreviations
- ADS:
-
approximate diameter of soma
- AG(1,-7):
-
abdominal ganglia (unfused abdominal neuromeres)
- Br:
-
brain
- N1–5:
-
lateral segmental nerves
- NCC1+2:
-
(medial) nerve 1+2 of corpus cardiacum
- NCC2:
-
nerve 2 of corpus cardiacum
- SOG:
-
subesophageal ganglion
- TAG:
-
terminal abdominal ganglion
- TG(1, 2, 3):
-
thoracic ganglia (unfused thoracic neuromeres)
- TG3+AG1–3:
-
fused metathoracic and abdominal ganglia 1–3
- TG2–3+AGn:
-
terminal ganglion of P. apterus
References
Agricola H-J, Bräunig P (1995) Comparative aspects of peptidergic signaling pathways in the nervous systems of arthropods. In: Breidbach O, Kutsch W (eds) The nervous systems of invertebrates: an evolutionary and comparative approach. Birkhäuser, Basel, pp 303–327
Baggerman G, Clynen E, Mazibur R, Veelaert D, Breuer M, De Loof A, Tanaka S, Schooffs L (2001) Mass spectrometric evidence for the deficiency in the dark-color-inducing hormone, [His7]-corazonin in an albino strain of Locusta migratoria as well as for its presence in solitary Schistocerca gregaria. Arch Insect Biochem Physiol 47:150–160
Cantera R, Veenstra JA, Nässel DR (1994) Postembryonic development of corazonin-containing neurons and neurosecretory cells in the blowfly, Phormia terraenovae. J Comp Neurol 350:559–572
Copenhaver PF, Truman JW (1986) Metamorphosis of the cerebral neuroendocrine system in the moth Manduca sexta. J Comp Neurol 249:186–204
Gäde G, Hoffmann K-H, Spring JH (1997) Hormonal regulation in insects: facts, gaps, and future directions. Physiol Rev 44:963–1032
Hansen IA, Sehnal F, Meyer SR, Scheller K (2001) Corazonin gene expression in the waxmoth Galleria mellonella. Insect Mol Biol 10:341–346
Homberg U, Davis NT, Hildebrand JG (1991) Peptide immunocytochemistry of neurosecretory cells in the brain and retrocerebral complex of the sphinx moth Manduca sexta. J Comp Neurol 303:35–52
Hua Y-J, Ishibashi J, Saito H, Tawfik AI, Sakakibara M, Tanaka Y, Derua R, Waelkens E, Baggerman G, De Loof A, Schoofs L, Tanaka S (2000) Identification of [Arg7] corazonin in the silkworm, Bombyx mori, and the cricket, Gryllus bimaculatus, as a factor inducing dark color in an albino strain of the locust, Locusta migratoria. J Insect Physiol 46:853–859
Nijhout HF (1975) Axonal pathways in the brain-retrocerebral complex of Manduca sexta (L.) (Lepidoptera: Sphingidae). Int J Insect Morphol Embryol 4:529–538
Petri B, Stengl M, Würden S, Homberg U (1995) Immunocytochemical characterization of the accessory medulla in the cockroach Leucophaea maderae. Cell Tissue Res 282:3–19
Predel R (2001) Peptidergic neurohemal system of an insect: mass spectrometric morphology. J Comp Neurol 436:363–375
Predel R, Agricola H, Linde D, Wollweber L, Veenstra JA, Penzlin H (1994) The insect neuropeptide corazonin: physiological and immunocytochemical studies in Blattariae. Zoology 98:35–49
Predel R, Kellner R, Gäde G (1999) Myotropic neuropeptides from the retrocerebral complex of the stick insect, Carausius morosus (Phasmatodea: Lonchodidae). Eur J Entomol 96:275–278
Predel R, Rapus J, Eckert M (2001) Myoinhibitory neuropeptides in the American cockroach. Peptides 22:199–208
Šauman I, Reppert SM (1996) Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of period protein regulation. Neuron 17:889–900
Schoofs L, Baggerman G, Veelaert D, Breuer M, Tanaka S, De Loof A (2000) The pigmentotropic hormone [His7]-corazonin, absent in a Locusta migratoria albino strain, occurs in an albino strain of Schistocerca gregaria. Mol Cell Endocrinol 168:101–109
Tanaka S (1993) Hormonal deficiency causing albinism in Locusta migratoria. Zool Sci 10:467–471
Tanaka S (1996) A cricket (Gryllus bimaculatus) neuropeptide induces dark colour in the locust, Locusta migratoria. J Insect Physiol 42:287–294
Tanaka S (2000) Induction of darkening by corazonins in several species of Orthoptera and their possible presence in ten insect orders. Appl Entomol Zool 35:509–517
Tanaka S (2001) Endocrine mechanisms controlling body-color polymorphism in locusts. Arch Insect Biochem Physiol 47:139–149
Tanaka S, Pener MP (1994) A neuropeptide controlling the dark pigmentation in color polymorphism of the migratory locust, Locusta migratoria. J Insect Physiol 40:997–1005
Tanaka S, Zhu D-H, Hoste B, Breuer M (2002) The dark-color inducing neuropeptide, [His7]-corazonin, causes a shift in morphometric characteristics towards the gregarious phase in isolated-reared (solitarious) Locusta migratoria. J Insect Physiol 48:1065–1074
Tanaka Y, Hua YJ, Roller L, Tanaka S (2002) Corazonin reduces the spinning rate in the silkworm, Bombyx mori. J Insect Physiol 48:707–715
Tawfik IA, Tanaka S, De Loof A, Schoofs L, Baggerman G, Waelkens E, Derura R, Milner Y, Yerushalmi Y, Pener MP (1999) Identification of the gragarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc Natl Acad Sci U S A 96:7083–7087
Veenstra J (1989) Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett 250:231–234
Veenstra J (1991) Presence of corazonin in three insect species, and isolation and identification of [His7] corazonin from Schistocerca americana. Peptides 12:1285–1289
Veenstra J (1994) Isolation and structure of the Drosophila corazonin gene. Biochem Biophys Res Comm 204:292–296
Veenstra JA, Davis NT (1993) Localization of corazonin in the nervous system of the cockroach Periplaneta americana. Cell Tissue Res 274:57–64
Žitňan D, Kingan TG, Kramer SJ, Beckage NE (1995) Accumulation of neuropeptides in the cerebral neurosecretory system of Manduca sexta larvae parasitized by the braconid wasp Cotesia congregata. J Comp Neurol 356:83–100
Acknowledgements
The authors would like to thank Dr. D. Žitňan (Bratislava) for methodical advice on whole-mount immunohistochemistry and critical comments on the manuscript, Prof. Dr. J. Veenstra (France) for kindly providing antibody against corazonin, and Ms. F. Yukuhiro and Dr. T. Kotaki (IIAS) for technical advice. Laboratory assistance was kindly provided by Ms. N. Kemmochi, Ms. S. Ogawa and Ms. H. Ikeda (IIAS). We also thank Dr. T. Kotaki, Dr. Y. Hirai and Dr. M. Hatakeyama (IIAS) for supplying the insects C. lineata, A. cuprea and A. rosae, respectively. The grasses used for locust rearing were raised by the Field Management Section of IIAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was partly supported by the Science and Technology Agency (STA fellowship, ID No. 200146), Japan
Rights and permissions
About this article
Cite this article
Roller, L., Tanaka, Y. & Tanaka, S. Corazonin and corazonin-like substances in the central nervous system of the Pterygote and Apterygote insects. Cell Tissue Res 312, 393–406 (2003). https://doi.org/10.1007/s00441-003-0722-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0722-4