Abstract
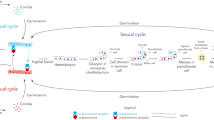
The availability of cloned genes that control sexual reproduction (mating type genes) in higher fungi has allowed us to consider the causes of failure to mate in asexual fungi. We report here that the asexual fungusBipolaris sacchari has a homolog of theMAT-2 gene of its sexual ascomycete relativeCochliobolus heterostrophus. TheB. sacchari MAT-2 sequence is highly similar to that ofC. heterostrophus MAT-2 and, in fact, functions in transgenicC. heterostrophus. Thus, the asexual nature ofB. sacchari is not due to absence or mutation ofMAT. When either of theC. heterostrophus MAT genes was transformed intoB. sacchari, the recipient could neither self nor cross with otherB. sacchari strains, in contrast to transgenicC. heterostrophus strains which can do both. Persistent asexuality ofB. sacchari, in spite of the presence of complementary functionalMAT genes, suggests that this fungus lacks genes other thanMAT which are essential for mating. Notably, the transgenicB. sacchari strains were sometimes able to initiate, but not complete, sexual development in interspecific pairings withC. heterostrophus. Transcript analysis showed that theB. sacchari MAT-2 gene is expressed in transgenicC. heterostrophus and that theC. heterostrophus MAT genes are expressed in transgenicB. sacchari. No transcript of the nativeB. sacchari MAT-2 gene was detected under any growth condition tested.
Similar content being viewed by others
References
Alcorn JL (1988) The taxonomy of “Helminthosporium” species. Annu Rev Phytopathol 26:37–56
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arnaise S, Zickler D, Glass NL (1993) Heterologous expression of mating-type genes in filamentous fungi. Proc Natl Acad Sci USA 90:6616–6620
Berbee ML, Taylor JW (1993) Ascomycete relationships: dating the origin of asexual lineages with 18S ribosomal RNA gene sequence data. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK, pp 67–78
Bronson CR (1988) Ascospore abortion in crosses ofCochliobolus heterostrophus heterozygous for the virulence locusTox1. Genome 30:12–18
Christiansen SK, Sharon A, Yoder OC, Turgeon BG (1993) Functional conservation of mating type genes and interspecific mating. Proc 17th Fungal Genetics Conference, Asilomar, Calif.
Coppin E, Arnaise S, Contamine V, Picard M (1993) Deletion of the mating-type sequences inPodospora anserina abolishes mating without affecting vegetative functions and sexual differentiation. Mol Gen Genet 241:409–414
Debuchy R, Coppin E (1992) The mating types ofPodospora anserina—functional analysis and sequence of the fertilization domains. Mol Gen Genet 233:113–121
Debuchy R, Arnaise S, Lecellier G (1993) Themat— allele ofPodospora anserina contains three regulatory genes required for the development of fertilized female organs. Mol Gen Genet 241:667–673
Egel R, Nielsen O, Weilguny D (1990) Sexual differentiation in fission yeast. Trends Genet 6:369–373
Ferreira, Glass (1995) Expression of three genes of theNeurospora crassa A idiomorph in the vegetative and sexual phases. Proc 18th Fungal Genet Conf, p 75. Asilomar CA
Glass NL, Grotelueschen J, Metzenberg RL (1990)Neurospora crassa A mating-type region. Proc Natl Acad Sci 87:4912–4916
Glass NL, Vollmer SJ, Staben C, Grotelueschen J, Metzenberg RL, Yanofsky C (1988) DNAs of the two mating-type alleles ofNeurospora crassa are highly dissimilar. Science 241:570–573
Greuter W (1988) Regnum Vegetabile, Vol. 118. International Code of Botanical Nomenclature. Fourteenth International Botanical Congress, Berlin, West Germany. Koeltz Scientific Books, Koenigstein, West Germany
Griess EA, Rensing LA, Grasser KD, Maier UG, Feix G (1993) Phylogenetic relationships of HMG box DNA-binding domains. J Mol Evol 37:204–210
Herskowitz I (1989) A regulatory hierarchy for cell specialization in yeast. Nature 342:749–757
Hicks J, Strathern JN, Klar AJS (1979) Transposable mating-type genes inSaccharomyces cerevisiae. Nature 282:478–483
Kelly M, Burke J, Smith M, Klar A, Beach D (1988) Four mating-type genes control sexual differentiation in the fission yeast. EMBO J 7:1537–1548
Leach J, Lang BR, Yoder OC (1982) Methods for selection of mutants and in vitro culture ofCochliobolus heterostrophus. J Gen Microbiol 128:1719–1729
Leonard KJ (1972) Formation of protothecia byCochliobolus heterostrophus. Mycologia 64:437–441
Macko V (1983) Structural aspects of toxins. In: Daly JM, Deverall BJ (ed) Toxins and plant pathogenesis. Academic Press, Sydney, pp 41–80
Metzenberg RL, Glass NL (1990) Mating type and mating strategies inNeurospora. Bioessays 12:53–59
Nelson RR (1960) A correlation of interspecific fertility and conidial morphology in species ofHelminthosporium exhibiting bipolar germination. Mycologia 52:753–761
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci 85:2444–2448
Philley ML, Staben C (1994) Functional analyses of theNeurospora crassa MT a-1 mating type polypeptide. Genetics 137:715–722
Picard M, Debuchy R, Coppin E (1991) Cloning the mating types of the heterothallic fungusPodospora anserina—developmental features of haploid transformants carrying both mating types. Genetics 128:539–547
Reynolds DR, Taylor JW (1992) Article 59: reinterpretation or revision? Taxon 41:91–98
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a laboratory manual, 2nd (edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Matthijs JS, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PM (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA binding motif. Nature 346:240–244
Sivanesan A (1987) Graminicolous species ofBipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. CAB International, Wallingford, UK
Staben C, Yanofsky C (1990)Neurospora crassa a mating type region. Proc Natl Acad Sci 87:4917–4921
Turgeon BG, Bohlmann H, Ciuffetti LM, Christiansen SK, Yang G, Schafer W, Yoder OC (1993a) Cloning and analysis of the mating-type genes fromCochliobolus heterostrophus. Mol Gen Genet 238:270–284
Turgeon BG, Christiansen SK, Yoder OC (1993b) Mating-type genes in Ascomycetes and their Imperfect relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK, pp 199–215
Turgeon BG, Garber RC, Yoder OC (1985) Transformation of the fungal maize pathogenCochliobolus heterostrophus using theAspergillus nidulans amdS gene. Mol Gen Genet 201:450–453
Turgeon BG, Sharon A, Wirsel S, Christiansen SK, Yamaguchi K, Yoder OC (1995) Structure and function of mating type genes inCochliobolus spp. and asexual fungi. Can J Bot 73:S778-S783
Tzeng TH, Lyngholm LK, Ford CF, Bronson CR (1992) A restriction fragment length polymorphism map and electrophoretic karyotype of the fungal maize pathogenCochliobolus heterostrophus. Genetics 130:81–96
VanWert SL, Yoder OC (1992) Structure of theCochliobolus heterostrophus glyceraldehyde-3-phosphate dehydrogenase gene. Curr Genet 22:29–35
Yang G, Turgeon BG, Yoder OC (1994) Toxin-deficient mutants from a toxin-sensitive transformant ofCochliobolus heterostrophus. Genetics 137:751–757
Yoder OC (1988)Cochliobolus heterostrophus, cause of Southern corn leaf blight. In: Sidhu GS (ed) Genetics of plant pathogenic fungi. Academic Press, San Diego, pp 93–112
Author information
Authors and Affiliations
Additional information
Communicated by C. A. M. J. J. van den Hondel
Rights and permissions
About this article
Cite this article
Sharon, A., Yamaguchi, K., Christiansen, S. et al. An asexual fungus has the potential for sexual development. Molec. Gen. Genet. 251, 60–68 (1996). https://doi.org/10.1007/BF02174345
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02174345